Repair of type A dissection-benefits of dissection rota
Introduction
Mortality and morbidity from an acute type A aortic dissection remains high despite technical improvements and is still in the range of 10–30% (1-6). The International Registry of Acute Dissection (IRAD) has published outcomes from multiple centres worldwide, with an average mortality of 25.1% in 2005 (5). European registries in the UK and Germany have published operative mortalities of 23.1% and 17% respectively (7,8). A recent publication from the Mount Sinai Medical Centre, using the Nationwide Inpatient Sample database of 24,777 patients between 1998 and 2008, showed an average operative mortality of 21.6% (9). Further analysis of this data set demonstrated that mortality was related to surgeon volume (odds ratio, 1.78) and centre volume. The relationship between volume and outcomes has been demonstrated in many surgical specialties and certainly in the United Kingdom has led to structural reorganization of general vascular services in an attempt to improve outcomes (10).
Following pursuit, at the Liverpool Heart and Chest Hospital, a dissection rota was established in 2007 in response to perceived poor outcomes from acute type A aortic dissection repair. The primary aim was to reduce operative mortality and associated morbidity and consequently improve long term survival. Prior to the change, all elective and emergency aortic surgeries were performed by nine general cardiac surgeons.
The purpose of this study is to investigate whether the acute type A dissection rota has improved clinical outcomes and survival.
Methods
Study population
This study collected data on all patients who had undergone emergency surgery for acute type A aortic dissection repair (ATADR) at Liverpool Heart and Chest Hospital between October 1998 and November 2015. Patients were split into two groups based around the creation of an on-call rota at the beginning of September 2007. Prior to September 2007, ATADR was performed by nine surgeons on a general cardiac on call rota. Following this date, a subspecialist aortic on call dissection rota was established with four aortic consultants on the roster. These surgeons performed the vast majority of the elective and all of the non-elective thoracic aortic surgeries.
Data collection
All study data were prospectively entered into an electronic database by the operating surgeon during the study period. The database was validated retrospectively by case note review. Outcomes evaluated for the purposes of this study included key quality markers as defined by STS (Society of Thoracic Surgeons) for coronary surgery (11): in-hospital mortality, stroke, re-exploration for bleeding, renal failure and prolonged ventilation times.
Operative techniques
Our operative techniques have expectedly evolved and improved through the study period, as technology and experience have shaped our approach. All elective operations were performed through a midline and full sternotomy. In a very small number of emergencies we used lateral extension to a sternotomy incision. A myriad of cannulation techniques was used depending on the anatomy, pathology, clinical stability and available imaging. Arterial cannulation was performed either of the ascending aorta, arch of the aorta, innominate artery, femoral artery or axillary arteries. In a limited number of emergency operations, the left ventricular apex or true lumen of ascending aorta (using epiaortic ultrasound) were cannulated. All cannulations via the axillary artery were performed using an 8 mm Hemashield graft conduit. Venous drainage was achieved via the right atrial appendage, bicaval cannulation or femoral vein. Venting of the heart was either performed through right superior pulmonary vein, main pulmonary artery or LV apex via a mini left thoracotomy.
Cardiopulmonary bypass was instigated following full heparinization (300 U/kg) to an ACT greater than 450, and during active cooling, alpha stat was maintained. Warming was commenced, ensuring no excessive differential between peripheral and core temperatures. In the majority of cases, intermittent cold blood cardioplegia was administered antegrade at induction and retrograde during maintenance. Typically, antegrade cardioplegia was supplemented into the right coronary territory throughout. Our general approach has been to administer a “hot shot” of warm blood cardioplegia prior to reflow. A small number of cases were performed with cold crystalloid cardioplegia. It is our practice to monitor a radial and femoral arterial trace as well as central venous pressure. A nasopharyngeal temperature probe and bladder/rectal catheter are used to monitor brain and core temperature. Bispectral Index Monitoring (BIS™ Brain Monitoring System, Covidien, Medtronic) and Near Infrared Spectroscopy (NIRS, Thermo Scientific) are employed. Transesophageal echocardiography is routinely utilized unless contraindicated. Neuroprotection was achieved with deep hypothermic circulatory arrest however typical adjuncts include carbon dioxide flooding of operative field, packing of the head with ice, phenobarbitone and supplementary cerebral perfusion. Our core (urinary or rectal) target temperatures for dissection have evolved over the time of the study. During early periods target temperature for all procedures was less than 18 °C. Currently, our typical target for emergency cases is a core temperature of 20 °C. Both antegrade and retrograde cerebral perfusion were employed. Antegrade cerebral perfusion was typically, but not exclusively, administered during hypothermic circulatory arrest. Cold blood is administered either via the head and neck vessels directly, or via perfusing the axillary artery and clamping of the brachiocephalic artery. The left subclavian artery is typically temporarily occluded but may be perfused. Target flows of 10 mls/kg/min are used; however, this is modified according to perfusion pressure and NIRS response. Retrograde cerebral perfusion was typically used for simple hemiarch surgery or acute pathologies. The SVC is cannulated with a 15F Retrograde Cannula and a small clamp placed between SVC and right atrial appendage. Flow is commenced at 10 mls/kg/min aiming for a CVP between 25–50 mmHg and an acceptable NIRS reading.
Statistical analysis
Due to non-normal distributions (assessed using the Kolmogorov–Smirnov test), continuous variables are shown as medians with 25th and 75th percentiles and comparisons were made with Wilcoxon rank-sum tests. In the matched pairs, comparisons were made with Wilcoxon signed rank tests. Categorical variables are shown as absolute frequencies with percentages. Unmatched comparisons were made with chi-squared tests or Fisher’s exact tests as appropriate, while matched comparisons were made with symmetry McNemar’s Chi square tests.
To account for differences in case-mix, we developed a propensity score for post-dissection rota group membership (12). The propensity for post-dissection rota group membership was determined without regard to outcome, using multivariable logistic regression analysis (13). A full non-parsimonious model was developed that included the 17 disaggregated pre-operative variables and the operative extent variables listed in Table 1. These variables were judged to be most relevant, appropriate and extensive to achieve a closely comparative group.
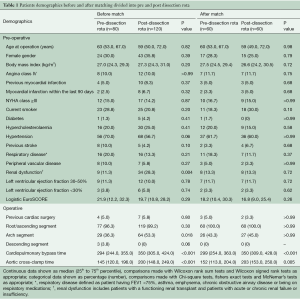
Full table
We then used a macro (full details available online at: http://www2.sas.com/proceedings/sugi29/165-29.pdf) to perform one-to-one propensity matching. This macro uses a greedy algorithm that matches without replacement. An eight-digit propensity score matching technique was performed; failing this, a seven, six, five, four, three, two or one digit match was set to be performed. The goal is to balance patient characteristics by incorporating everything recorded that may relate to either systematic bias or simply chance.
The Kaplan-Meier estimator was used to construct survival graphs for the pre- and post-dissection rota groups, with the log-rank test used to assess the equivalence of death rates between groups.
In all cases, a P value less than 0.05 was considered significant. All statistical analyses were performed with SAS for Windows Version 9.3 (SAS Institute, Cary, NC, USA).
Results
Patient characteristics
Two hundred ATADR patients were identified and included in the analysis. Patient preoperative and operative characteristics are shown in Table 1. In the unmatched groups, there was a higher incidence of renal dysfunction in the post-dissection rota era (P=0.004). Amongst the operative variables, cardiopulmonary bypass, aortic cross clamp time and surgery on the aortic arch were all significantly increased in the post-dissection rota era (P<0.001, P<0.001 and P=0.018, respectively). The propensity-matched analysis provided 60 patients from the post-dissection rota era successfully matched to 60 from the pre-dissection rota era. The patient characteristics of the propensity matched groups are also in Table 1, showing that both groups were well matched with respect to major preoperative characteristics such as age, gender, BMI, left ventricular function, previous cardiac surgery and comorbidities such as diabetes, peripheral vascular disease, and respiratory and renal dysfunction.
Patient outcomes
There was no difference in the extent of procedures performed pre- and post-dissection rota after matching. However, cardiopulmonary bypass times and aortic cross clamp times were still significantly longer in the matched post–dissection rota cohort. In-hospital outcomes are shown in Table 2. Patients who underwent ATADR in the post-dissection rota era were less likely to suffer in-hospital mortality in both the matched and unmatched groups (30% vs. 13.3%; P=0.004 and 28.3% vs. 11.7%; P=0.055, respectively). A similar improvement was shown in acute renal failure (26.3% vs. 14.2%; P=0.033 and 31.7% vs. 15.0%; P=0.044, respectively). Fewer patients suffered prolonged ventilation times in the unmatched post-dissection group (32.5% vs. 16.7%; P=0.009), and although rates for this outcome in the matched group were similar, the results were no longer significant at the 95% level (31.7% vs. 16.7%; P=0.081). There were no significant differences in any of the other in-hospital outcomes.
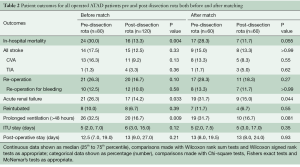
Full table
Survival
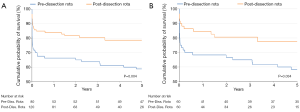
Mid-term survival is shown in Figure 1. We found a significant improvement in 5-year survival for the pre- and post-dissection rota in both the matched and unmatched patients (P=0.004 and P=0.034, Log-Rank test).
Discussion
Three acute type A aortic dissections are diagnosed out of every 1,000 emergency department patients presenting with acute back, chest, or abdominal pain (14). Mortality in untreated patients is estimated at more than 1% per hour after onset of symptoms, whereas 30-day survival for patients who are diagnosed early and treated appropriately approaches 80% (15,16). The International Registry for Acute Dissection and the Society for Cardiothoracic Surgery in Great Britain and Ireland published mortality rates of 26.6% and 25% respectively (1,2).
It is imperative to highlight that timely diagnosis and rapid surgical management of acute type A aortic dissection are of paramount importance for better outcomes and survival. Once diagnosed, the key to a successful outcome is rapid referral to a cardiac surgery center and immediate surgical intervention. Surgical outcomes are highly variable from center to center. The only published paper on standardization of care for aortic dissection comes from Minneapolis (11), where a regional protocol was instituted in August 2005. This began with clinical suspicion of the diagnosis in community hospitals, where a single telephone call activated the protocol, leading to operation by one of four specialist cardiovascular surgeons. The group demonstrated significantly reduced times from diagnosis to surgery but no significant reduction in mortality rates so far.
A good deal of literature exists relating outcome to volume of activity by surgeon and hospital in the related specialty of vascular surgery. Outcomes from the United States show a very clear relationship between activity and outcome from abdominal aortic aneurysm repair (17), and this has led to a major review in the UK and rearrangement of services (5) to address these issues and improve outcomes. Following pursuit, the Bristol Heart Institute published an analysis of their results for ascending/arch surgery in 2004 (18). The objective of the work was to compare outcomes within the unit between a single high volume operator and a group of other, more general operators performing aortic surgery on a more ad hoc basis. The study had a high percentage of urgent/emergency cases. Although there was no mortality difference between the two groups, there was a significant difference in morbidity.
The LHCH experience of dissection rota
Liverpool Heart and Chest Hospital (LHCH) covers a population of 2.8 million, performing 2,000 cardiac surgical procedure/year of which 180 cases are elective and non-elective thoracic and thoracoabdominal aortic aneurysm repairs. Despite the high volume of general cardiac surgery by 13 surgeons, it was felt that the hospital mortality for acute type A aortic dissection was excessive, at around 30%. Therefore, in 2007, LHCH became the first in the UK to implement a subspecialised on-call dissection rota for acute type A aortic dissection. This development was structured around multiple factors, least of which are the internal rearrangement of aortic services and innovations in aortic surgery, including advanced device technologies, anesthetic agents, perfusion techniques, brain protection methods, imaging modalities, neuromonitoring advances and post-operative monitoring techniques. The surgical and technical skills of the aortic team additionally contributed to the success of the subspecialised dissection rota. This evolution was initiated by a primary senior surgeon who aided in the development of this service and team and passed on the set of skills that were transferred amongst the team. It is inconceivable that such skills were further enhanced and undoubtedly correlated to the improved outcomes conveyed in this study.
Interestingly, while the extent of surgical intervention did not change between the two eras demonstrated in this study, the operating times, primarily cardiopulmonary bypass times and cross clamp times, was actually prolonged amongst the specialised team. The reasons behind this observation remain unclear but are likely due to the operative techniques developed to minimise bleeding and malperfusion, such as routine buttressing of suture lines. Whilst such techniques were more time-consuming, they clearly proved effective in improving surgical outcomes and overall survival.
Our results clearly demonstrate a reduction in mortality after acute type A aortic dissection, falling from 30% to 11.7% after implementation of the dissection rota. This improvement in survival is likewise demonstrated in the 5-year actuarial survival rates.
We acknowledge that this model of care is not appropriate to every hospital and that ours is one of the largest cardiothoracic units in the country. It may however, be regionally applied in order to provide consistent subspecialist out of hours, all year round.
Our study is obviously limited by its retrospective nature, but prospective randomised trials have never been performed in acute aortic dissection, and realistically are not likely. We acknowledge the potential developments in anesthetic and perfusion techniques over the last few years that all corresponded to improved outcomes observed following commencement of the dissection rota at LHCH. However, we feel that the development of standardized surgical techniques and the regular performance of these on a weekly basis as well as the increased number of cases per surgeon are amongst the predominant contributing factors to the benefits demonstrated, including improved midterm survival.
Limitations
Our study was conducted on a relatively small subset of patients, who have unique pathological characteristics. Furthermore, as the comparative groups are time-bound, there are many other potentially relevant factors that we could not consider, such as developments in theatre equipment, operational standards and the collective experience of the clinical staff in assessing and managing these patients.
It is difficult to account for these variables, particularly considering the small number of patients over such a period of time, and there is good evidence that several improvements in surgical techniques and organ protection have contributed to improved survival. Further study will be required to address the most effective approach for managing these patients.
Conclusions
The implementation of acute type A aortic dissection rota has resulted in significant improvement in hospital mortality and survival of our patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Committee for Scientific Affairs, Sakata R, Fujii Y, et al. Thoracic and cardiovascular surgery in Japan during 2009: annual report by the Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2011;59:636-67. [Crossref] [PubMed]
- Rampoldi V, Trimarchi S, Eagle KA, et al. Simple risk models to predict surgical mortality in acute type A aortic dissection: the International Registry of Acute Aortic Dissection score. Ann Thorac Surg 2007;83:55-61. [Crossref] [PubMed]
- Geirsson A, Szeto WY, Pochettino A, et al. Significance of malperfusion syndromes prior to contemporary surgical repair for acute type A dissection: outcomes and need for additional revascularizations. Eur J Cardiothorac Surg 2007;32:255-62. [Crossref] [PubMed]
- Chiappini B, Schepens M, Tan E, et al. Early and late outcomes of acute type A aortic dissection: analysis of risk factors in 487 consecutive patients. Eur Heart J 2005;26:180-6. [Crossref] [PubMed]
- Trimarchi S, Nienaber CA, Rampoldi V, et al. Contemporary results of surgery in acute type A aortic dissection: The International Registry of Acute Aortic Dissection experience. J Thorac Cardiovasc Surg 2005;129:112-22. [Crossref] [PubMed]
- Easo J, Weigang E, Hölzl PP, et al. Influence of operative strategy for the aortic arch in DeBakey type I aortic dissection: analysis of the German Registry for Acute Aortic Dissection Type A. J Thorac Cardiovasc Surg 2012;144:617-23. [Crossref] [PubMed]
- Conzelmann LO, Krüger T, Hoffmann I, et al. German Registry for Acute Aortic Dissection Type A (GERAADA): initial results. Herz 2011;36:513-24. [Crossref] [PubMed]
- Bridgewater B, Keogh B. Society for Cardiothoracic Surgery in Great Britain and Ireland, sixth adult cardiac surgical database report 2008, Demonstrating quality. Oxfordshire: Dendrite clinical systems Ltd. 2009. Available online: http://www.scts.org/_userfiles/resources/SixthNACSDreport2008withcovers.pdf
- Chikwe J, Cavallaro P, Itagaki S, et al. National outcomes in acute aortic dissection: influence of surgeon and institutional volume on operative mortality. Ann Thorac Surg 2013;95:1563-9. [Crossref] [PubMed]
- Karthikesalingam A, Hinchliffe RJ, Loftus IM, et al. Volume-outcome relationships in vascular surgery: the current status. J Endovasc Ther 2010;17:356-65. [Crossref] [PubMed]
- Shahian DM, Edwards F, Grover FL, et al. The Society of Thoracic Surgeons National Adult Cardiac Database: a continuing commitment to excellence. J Thorac Cardiovasc Surg 2010;140:955-9. [Crossref] [PubMed]
- Blackstone EH. Comparing apples and oranges. J Thorac Cardiovasc Surg 2002;123:8-15. [Crossref] [PubMed]
- Hosmer DW Jr, Lemeshow S. Applied Logistic Regression. New York: John Wiley & Sons Inc; 1989.
- Golledge J, Eagle KA. Acute aortic dissection. Lancet 2008;372:55-66. [Crossref] [PubMed]
- Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA 2000;283:897-903. [Crossref] [PubMed]
- Erbel R, Alfonso F, Boileau C, et al. Diagnosis and management of aortic dissection. Eur Heart J 2001;22:1642-81. [Crossref] [PubMed]
- Dillavou ED, Muluk SC, Makaroun MS. A decade of change in abdominal aortic aneurysm repair in the United States: Have we improved outcomes equally between men and women? J Vasc Surg 2006;43:230-8; discussion 238. [Crossref] [PubMed]
- Narayan P, Caputo M, Rogers CA, et al. Early and mid-term outcomes of surgery of the ascending aorta/arch: is there a relationship with caseload? Eur J Cardiothorac Surg 2004;25:676-82. [Crossref] [PubMed]





