Guilt by association: a paradigm for detection of silent aortic disease
Introduction
Thoracic aortic aneurysm (TAA) is a disease often referred to as a “silent killer” due its insidious and virulent nature (1). Indeed, in the absolute majority of patients, aneurysms of the thoracic aorta cause absolutely no symptoms (2). Often the first manifestation of TAA is either death, or a major complication that threatens to produce death (such as aortic rupture or dissection) unless an emergent surgical operation is performed (3).
In the United States alone, aneurysms of the aorta are responsible for approximately 13,000 deaths annually (4). This makes aortic aneurysm the 19th leading cause of death, and the 15th leading cause of death in individuals over 65 years (Table 1) (4). Please note that aortic aneurysms cause more deaths than the well-appreciated human immunodeficiency virus. However, even these numbers are likely underestimating the true burden of aortic disease on the survival of the population, because many aneurysm-related deaths are sudden in origin and, without a postmortem examination, are likely to be falsely classified as a “heart attack” (myocardial infarction) (5). This is supported by data from large-scale autopsy studies of sudden death patients, which show that aortic rupture and dissection accounts anywhere from 2% to 7.3% of all sudden deaths (6-8).
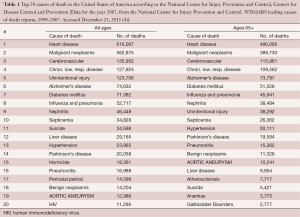
Full table
Aneurysms of the thoracic segment of the aorta are estimated to affect approximately 5–10 individuals per 100,000 people per year, as shown in two population-based studies (9,10). There is also evidence to suggest that the incidence of TAA is increasing with time (5), although it is not clear whether this is due to disease-specific properties or due to improvement in TAA detection modalities (advancements in imaging). Studies on the prevalence of TAA in the population (usually based on imaging a large volume of patients) have shown the prevalence of TAA to be between 0.16–0.34% (11,12). The validity of these prevalence studies is questionable, and these figures likely represent a significant underestimation of the true prevalence of TAA in the general population (since only aortas of 5 cm and greater were considered a TAA). A large-scale magnetic resonance imaging study from the Multi-Ethnic Study of Atherosclerosis (MESA) showed that out of 3,573 study participants 93 (2.6%) had an ascending aorta size between 4.0–4.4 cm, 7 participants (0.20%)—between 4.5 and 4.9 cm, and only one individual (0.03%) had an aorta of 5.0 cm (13,14).
The sobering lethality of TAA and the non-negligible incidence and prevalence figures underscore the importance of directing efforts in battling the “silent killer” towards early detection of individuals who either harbor an aneurysm in their chest or are at risk of developing one in the future. Once a TAA diagnosis is established, prophylactic surgical treatment can be safely performed for aneurysms of the ascending aorta, aortic arch, and descending/thoracoabdominal aorta (15), thus preventing aneurysm-related death.
The question is: how to detect individuals with (or at risk of developing) an asymptomatic disease? In this manuscript we review a paradigm for detection of individuals at risk of thoracic aortic disease that we call “Guilt by Association” (16), which is based on recognition of clinical conditions associated with thoracic aortic disease (Video 1).
The term “guilt by association”
Google defines the term “Guilt by Association” by the following: “guilt ascribed to someone not because of any evidence but because of their association with an offender”. This is very consonant with our efforts in identifying TAA via clinical associations with other known conditions. The term “Guilt by Association” has also been widely used in popular culture (novels by Susan R. Sloan and Marcia Clark, a 2002 movie by Graeme Campbell, and other works). We capitalize on this commonly used term and apply it to our paradigm of detecting aortic disease with hope that it will trigger an alert when one or more of the “associates” are discovered in a patient.
Guilt by association paradigm for TAA identification
The following clinical conditions/observations have been associated with thoracic aortic disease (Figure 1):
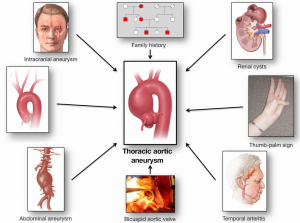
- Intracranial aneurysm;
- Aortic arch anomalies;
- Abdominal aortic aneurysm (AAA);
- Simple renal cysts (SRC);
- Bicuspid aortic valve;
- Family history of aortic disease;
- A positive thumb-palm sign;
- Temporal arteritis (and other autoimmune disorders).
We will review each one of these clinical markers one by one in the sections that follow.
Intracranial aneurysm
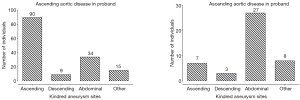
Cerebral aneurysms (Figure 2) are thought to share pathophysiologic features with TAA [including matrix metalloproteinase over-activity (17,18)]. In recent years a clinical association between these two conditions has been established as well. Patients with TAA have a 9% prevalence of intracranial aneurysm, which is nine-fold greater than in the general population (19). Furthermore, patients with descending aortic aneurysm have even a stronger correlation with intracranial aneurysm than patients with ascending aneurysm (33% vs. 7.1%, respectively) (Figure 3) (19). This association has also been studied in the opposite direction—approximately 5% of patients with intracranial aneurysm have a concomitant TAA (20), which again is significantly higher than the general population. Having discovered this association, we have made it a policy at our institution to image the brain of all patients prior to surgery on the thoracic aorta, and we obtain a neurosurgery consultation should a cerebral aneurysm be identified. Knowing about the association of TAA and intracranial aneurysm will help identify many patients with both—silent TAA and silent intracranial aneurysm, which may save many lives.
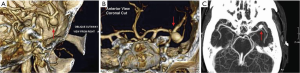

Aortic arch anomalies
The most commonly seen type of aortic arch branching anomalies is the bovine aortic arch (Figure 4). In patients with a bovine arch, the left common carotid artery takes off from the innominate artery (brachiocephalic trunk) instead of originating directly from the top of the aortic arch. It is important to note that bovine arch is a misnomer, since the anatomy of the cow’s aorta does not resemble this configuration. Previously, the bovine arch anomaly was considered to be completely benign. However, recent studies have shown that the frequency of bovine aortic arch is significantly increased in patients with thoracic aortic disease, compared to the general population (21-23). One study showed that in patients with TAA the frequency of bovine aortic arch was 20.7%, compared to 6.7% see in the non-TAA control group (Figure 5) (21).

Other arch anomalies, such as an isolated left vertebral artery and an aberrant right subclavian artery (Figure 4) have also been implicated with increased risk of thoracic aortic disease (23). In fact, analyzing all the aortic arch anomalies together, it appears that patients with thoracic aortic disease have a much lower frequency of normal aortic branching patterns compared to the normal population (23). Therefore, it appears that patients found to have an anomaly of the aortic arch branching pattern should be considered at higher risk for developing a TAA and should be imaged regularly.
Abdominal aortic aneurysm (AAA)
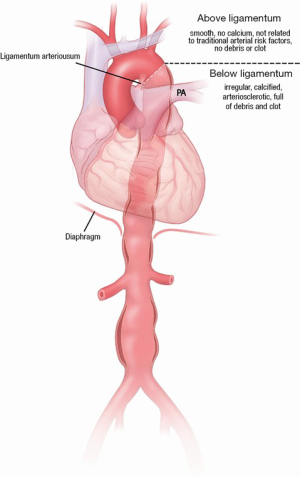
AAA have been well studied in terms of the approach for screening patients and surgical/endovascular management. Past studies have suggested that AAAs have much more in common with descending TAA (these are related to classic arteriosclerotic risk factors, such as hypertension, smoking, and dyslipidemia), while ascending aortic aneurysm are quite different, do not follow the classic arteriosclerotic risk factors, and are commonly and strongly genetic in origin (5). There appears to be a clear separation point between these two types of diseases at the ligamentum arteriosum (Figure 6) (5), which is also supported by the different embryologic origins of these segments of the aorta—the ascending aorta and arch originate from the neural crest, while the descending thoracic and abdominal aorta develop from the mesoderm (24-27). However, until recently it was not known whether patients with AAA were also at risk of developing a TAA. Recent studies have shown that 20–28% of patients with AAA also have or develop a TAA (28-30). This underlines the importance of screening the entire aorta in patients in whom one diseased segment has been identified.
Simple renal cysts (SRC)
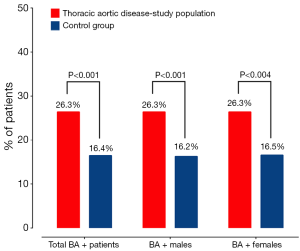
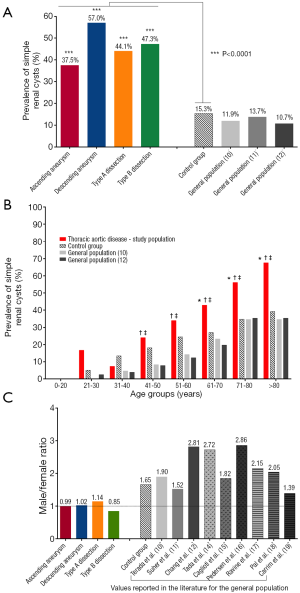
SRC, a common finding on imaging scans, have well-described natural history patterns. The reported prevalence of SRC in the general population ranges from 5–41%, with a weighted average of 12.6%. The number of patients with SRC increases with increasing age of patients. Twice as many males have SRC compared to females. Two separate studies have shown an association of SRCs with AAA (31) and aortic dissection (32). Another study found a higher prevalence of renal cysts in patients with Marfan syndrome compared to non-Marfan controls (33). A study from our group evaluated the frequency of SRC in patients with ascending and descending aortic aneurysm, as well as Type A and Type B dissection (34). We found that in all four of these groups of patients with thoracic aortic disease, the prevalence of SRC was significantly higher than in our control group and higher than reported for the general population (Figure 7A) (34). The prevalence of SRC was also higher in the thoracic aortic disease group when broken down by age groups, a valuable observation since age is an important contributor to the natural history of SRC (Figure 7B) (34). Moreover, the male preponderance for SRC that is seen in the general population (on average 2:1 male to female ratio) is lost in all four groups of patients with disease of the thoracic aorta (Figure 7C) (34). We feel that this association of TAA and SRC can potentially be explained by increased MMP activity in both conditions [TAA are associated with increased MMP (17) activity and high concentrations of MMPs have been shown to be present in the cystic fluid of benign SRC (35)], suggestive of a possible common genetic abnormality. Given such strong correlation of SRC with TAA, AAA and aortic dissection, it is advisable to consider screening patients with SRCs for aortic disease.
Bicuspid aortic valve
The most common congenital cardiac defect is a bicuspid aortic valve (BAV). According to population-based studies, a BAV is estimated to be present in 1–2% of the population (36-38). Concomitant bicuspid aortopathy is common as well, although the exact estimates vary greatly from 20% to 84% (Figure 8) (38). Previously, bicuspid aortopathy was thought to be more malignant than “regular” TAA cases, but not as severe as in patients with Marfan syndrome. Thus, some people referred to bicuspid aortopathy as “Marfan’s Light”. However, the evidence appears to be equivocal. One study has shown that bicuspid ascending aneurysms grow faster than aneurysms in patients with trileaflet aortic valves (0.19 vs. 0.13 cm/year, respectively) (39). It is important to remember that BAV-related aortic aneurysms are a major causative factor for aortic dissection, which usually occurs long before a clinically evident aortic stenosis will develop (37). At the same time, some recent studies have shown that the long-term survival for patients with a BAV is not much different from a matched control population (40). However, we still recommend close attention to be paid to the ascending aorta (and probably other segments of the aorta as well) once a BAV has been identified in a patient.
Family history of aortic disease
For many years, Marfan syndrome was the only link of thoracic aortic disease to genetics, since TAA and dissection are well-known manifestations of Marfan’s disease. However, it soon became apparent that Marfan syndrome explains less than 5% of all cases of TAA (41,42), and even together with all other discovered syndromic connective tissue disorders (Ehlers-Danlos syndrome, Loeys-Dietz syndrome, Turner syndrome) still accounts for only a very small percentage of all cases of TAA.
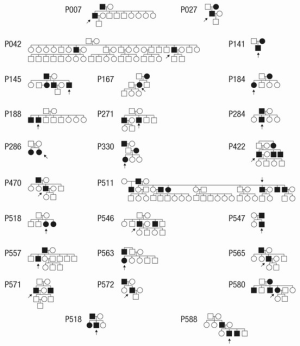
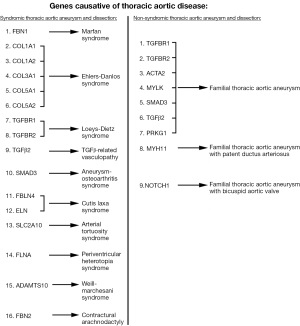
Since the late 1990s, evidence has been accumulating in support of the genetic nature of thoracic aortic disease and the importance of accurate family history assessment in patients. The initial studies (conducted by analyzing Mendelian patterns of inheritance) showed that 21% of patients with thoracic aortic disease have at least one or more relatives affected with aneurysm disease (Figure 9) (43,44). This was the first recognition that genetics play an important role in non-syndromic TAA and their transmission. Further in-depth studies have shown that the main mode of inheritance of thoracic aortic disease is autosomal dominant, although other modes of inheritance are also observed (autosomal recessive and X-linked) (45). Another interesting finding was the observation that patients with ascending aneurysm were more likely to have a relative with an ascending aortic aneurysm/dissection, while patients with aneurysms of the descending thoracic aorta were more likely to have kindred with AAA (Figure 10) (45). Importantly, familial TAA patients tend to present at a younger age than non-familial sporadic patients (58 vs. 66 years, respectively), and familial aortas grow faster as well (0.21 vs. 0.16 cm/y) (45). These studies led to the acknowledgement of the importance of family history of thoracic aortic disease in the US (46) and European (47) Guidelines on management of thoracic aortic pathology. The Guidelines currently recommend aortic imaging for first-degree relatives of patients with TAA and/or dissection to identify those with asymptomatic disease (Class I, Level of Evidence B), and if one or more of the first-degree relatives is found to have thoracic aortic disease, then aortic imaging for second-degree relatives is recommended as well (Class IIa, Level of Evidence B) (46). However, aortic imaging will only show the presence of absence of aortic disease at a given time, so individuals with normal sized aortas on an initial scan cannot be certain that the disease will not develop in the years to come [TAAs are virulent, but indolent at the same time and often take years, even decades, to grow to a sizable dimension (5)]. This is where modern genetic testing techniques, such as next-generation sequencing, are particularly useful and can screen a patient for all genes known to cause thoracic aortic disease. Currently, as this paper is being written, 21 genes have been proven to cause syndromic and non-syndromic thoracic aortic disease (Figure 11), and new genes are being discovered regularly (48).
So, over the years our understanding of the genetic nature of thoracic aortic diseases has evolved significantly. However, the importance and simplicity of taking an accurate family history during a patient-physician encounter is hard to over-estimate. This is one of the most important tools that we have to identify silent aneurysms and individuals at risk of developing aneurysms in the future.
A positive thumb-palm sign
The ability of a person to cross the thumb beyond the ulnar border of the flat palm (as shown in Figure 12) is considered a positive thumb-palm sign. This simple sign has long been considered characteristic of Marfan syndrome (49) and is included as one of the diagnostic criteria in the revised Ghent nosology for Marfan’s disease (50). However, the thumb-palm sign is also useful in detecting other connective tissue disorders associated with TAA. A positive thumb-palm sign is an indicator that the bones are excessively long and the joints are lax, common manifestations of connective tissue disease (5). In fact, in some individuals a positive thumb-palm sign will be the only manifestation that triggers a search for aneurysm in the absence of other stigmata (5). Although the sensitivity and specificity of the thumb-palm sign are not currently known, we highly recommend applying this simple test to individuals thought to be at risk for aneurysm disease. A negative thumb-palm sign will not rule out the possibility of having a TAA, but a positive thumb-palm sign should trigger a search for thoracic aortic disease.

Temporal arteritis (and other autoimmune disorders)
Giant cell (temporal) arteritis and other autoimmune disorders have been associated with TAA (51-53). A population-based cohort study from Olmstead County (Minnesota) showed that patients with temporal arteritis were 17 times more likely to develop a TAA than patients without this condition (54). A systematic review and meta-analysis showed that TAA developed in 2–8% of patients with temporal arteritis (55). Therefore, it is advisable to initiate a search for TAA in a patient diagnosed with giant cell arteritis or other autoimmune disorders.
Other potential clinical markers of thoracic aortic disease
In the sections above we have described in detail eight of the most common and well-recognized clinical markers of thoracic aortic disease. However, other markers exist as well, some of which have been shown to be associated with TAA, and some yet to be investigated and confirmed.
Inguinal hernias: one recent study from Sweden showed an association of thoracic aortic disease and inguinal hernia (56). The prevalence of TAA in patients with inguinal hernias was shown to be much higher than in the general population (56).
Liver cysts: together with renal cysts, cysts in the liver have been shown to be increased in prevalence in patients with Marfan syndrome (33). This is a marker that is worth studying in non-syndromic TAA patients as well.
Polycystic kidney disease: above we discussed SRC. Polycystic kidney disease, as well, has been associated with aneurysms in various vascular beds, including the abdominal aorta and intracranial vasculature. However, evidence linking polycystic kidney disease with thoracic aortic disease is anecdotal and is limited to several case reports (57-59). This association requires further in-depth analysis.
Hypothyroidism: there is evidence to suggest that in patients with hypothyroidism the arterial stiffness (including that of the thoracic aorta) is increased (60,61). Clinical data are limited to a few case reports on patients with thoracic aortic disease and hypothyroidism (62,63). This association too requires additional large-scale studies to prove its veracity.
Future development
Timely identification of individuals with TAA or at risk of developing TAA is a challenging, but very important task, given how lethal this disease can be when it strikes. At the same time, given its relative rarity, widespread radiographic screening for TAA has not been shown to be cost-effective. Therefore, for a number of years investigators have been in a search of biomarkers that could detect an aneurysm and predict (and thus prevent) the onset of aortic dissection (64,65). However, the trouble with existing biomarkers is that they are detectable only post-dissection, but cannot detect a silent aneurysm or a looming dissection episode. Future developments in this direction should be aimed at identifying a pre-dissection biomarker.
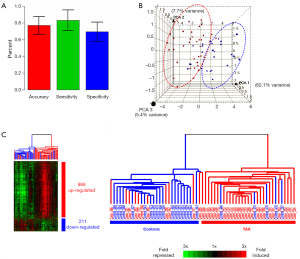
At our institution, we have investigated the possibility of using a ribonucleic acid (RNA)-signature test as a potential biomarker and screening tool. This RNA-signature was developed by studying 30,000 RNA expression patterns, among which a 41 RNA panel was found to be highly effective at identifying patients with a TAA—from a simple blood test (66). The overall accuracy of this test was approximately 78%, with a sensitivity of 81% and a specificity of 75% (Figure 13) (66). The clustering diagram depicted in Figure 13 illustrates well the accuracy of this test. This RNA-signature test has been replicated twice in different populations of TAA patients, and in patients with Marfan syndrome and acute aortic dissection—with very favorable results. We believe that the RNA-signature test has the potential to become a useful biomarker for thoracic aortic disease, which could be relatively inexpensive and suitable for widespread application.
Conclusions
In conclusion, through these clinical observations and association studies, we hope to provide physicians and practitioners with a valuable tool-kit for early identification of clinically silent TAA by applying the Guilt by Association principle (Figure 1). Once a TAA is detected in a patient, regular imaging and well-timed prophylactic surgical correction will most certainly contribute to lowering the mortality burden of this disease.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Elefteriades JA. Beating a sudden killer. Sci Am 2005;293:64-71. [Crossref] [PubMed]
- Elefteriades JA. Thoracic aortic aneurysm: reading the enemy's playbook. Curr Probl Cardiol 2008;33:203-77. [Crossref] [PubMed]
- Ziganshin BA, Elefteriades JA. Thoracic Aortic Disease. In: Stergiopoulos K, Brown DL, eds. Evidence-Based Cardiology Consult.1st ed. London: Springer-Verlag; 2014:331-53.
- WISQARS leading causes of death reports, 1999-2007. 2015. Accessed November 15, 2015. Available online: http://webappa.cdc.gov/sasweb/ncipc/leadcaus10.html
- Elefteriades JA, Farkas EA. Thoracic aortic aneurysm clinically pertinent controversies and uncertainties. J Am Coll Cardiol 2010;55:841-57. [Crossref] [PubMed]
- Murai T. Aortic dissection and sudden death--statistical analysis on 1320 cases autopsied at Tokyo-to Medical Examiner Office. Nihon Hoigaku Zasshi 1988;42:564-77. [PubMed]
- Nagata M, Ninomiya T, Doi Y, et al. Temporal trends in sudden unexpected death in a general population: the Hisayama study. Am Heart J 2013;165:932-8. [Crossref] [PubMed]
- di Gioia CR, Autore C, Romeo DM, et al. Sudden cardiac death in younger adults: autopsy diagnosis as a tool for preventive medicine. Hum Pathol 2006;37:794-801. [Crossref] [PubMed]
- Bickerstaff LK, Pairolero PC, Hollier LH, et al. Thoracic aortic aneurysms: a population-based study. Surgery 1982;92:1103-8. [PubMed]
- Clouse WD, Hallett JW Jr, Schaff HV, et al. 3rd. Improved prognosis of thoracic aortic aneurysms: a population-based study. JAMA 1998;280:1926-9. [Crossref] [PubMed]
- Itani Y, Watanabe S, Masuda Y, et al. Measurement of aortic diameters and detection of asymptomatic aortic aneurysms in a mass screening program using a mobile helical computed tomography unit. Heart Vessels 2002;16:42-5. [Crossref] [PubMed]
- Kälsch H, Lehmann N, Möhlenkamp S, et al. Body-surface adjusted aortic reference diameters for improved identification of patients with thoracic aortic aneurysms: results from the population-based Heinz Nixdorf Recall study. Int J Cardiol 2013;163:72-8. [Crossref] [PubMed]
- Turkbey EB, Jain A, Johnson C, et al. Determinants and normal values of ascending aortic diameter by age, gender, and race/ethnicity in the Multi-Ethnic Study of Atherosclerosis (MESA). J Magn Reson Imaging 2014;39:360-8. [Crossref] [PubMed]
- Paruchuri V, Salhab KF, Kuzmik G, et al. Aortic Size Distribution in the General Population: Explaining the Size Paradox in Aortic Dissection. Cardiology 2015;131:265-72. [Crossref] [PubMed]
- Achneck HE, Rizzo JA, Tranquilli M, et al. Safety of thoracic aortic surgery in the present era. Ann Thorac Surg 2007;84:1180-5; discussion 1185. [Crossref] [PubMed]
- Elefteriades JA, Sang A, Kuzmik G, et al. Guilt by association: paradigm for detecting a silent killer (thoracic aortic aneurysm). Open Heart 2015;2:e000169. [Crossref] [PubMed]
- Kuzmik GA, Feldman M, Tranquilli M, et al. Concurrent intracranial and thoracic aortic aneurysms. Am J Cardiol 2010;105:417-20. [Crossref] [PubMed]
- Koullias GJ, Ravichandran P, Korkolis DP, et al. Increased tissue microarray matrix metalloproteinase expression favors proteolysis in thoracic aortic aneurysms and dissections. Ann Thorac Surg 2004;78:2106-10; discussion 2110-1. [Crossref] [PubMed]
- Kim SC, Singh M, Huang J, et al. Matrix metalloproteinase-9 in cerebral aneurysms. Neurosurgery 1997;41:642-66; discussion 646-7. [PubMed]
- Kuzmik GA, Gunel M, Bulsara KR, et al. Intracranial Aneurysm Patients May Harbor Thoracic Aortic Aneurysms. 2012. Available online: http://aats.org/aortic/abstracts/2012/303.cgi
- Dumfarth J, Chou AS, Ziganshin BA, et al. Atypical aortic arch branching variants: A novel marker for thoracic aortic disease. J Thorac Cardiovasc Surg 2015;149:1586-92. [Crossref] [PubMed]
- Hornick M, Moomiaie R, Mojibian H, et al. 'Bovine' Aortic Arch - A Marker for Thoracic Aortic Disease. Cardiology 2012;123:116-24. [Crossref] [PubMed]
- Malone CD, Urbania TH, Crook SE, et al. Bovine aortic arch: a novel association with thoracic aortic dilation. Clin Radiol 2012;67:28-31. [Crossref] [PubMed]
- Jiang X, Rowitch DH, Soriano P, et al. Fate of the mammalian cardiac neural crest. Development 2000;127:1607-16. [PubMed]
- Cheung C, Bernardo AS, Trotter MW, et al. Generation of human vascular smooth muscle subtypes provides insight into embryological origin-dependent disease susceptibility. Nat Biotechnol 2012;30:165-73. [Crossref] [PubMed]
- Gittenberger-de Groot AC, DeRuiter MC, Bergwerff M, et al. Smooth muscle cell origin and its relation to heterogeneity in development and disease. Arterioscler Thromb Vasc Biol 1999;19:1589-94. [Crossref] [PubMed]
- Ruddy JM, Jones JA, Ikonomidis JS. Pathophysiology of thoracic aortic aneurysm (TAA): is it not one uniform aorta? Role of embryologic origin. Prog Cardiovasc Dis 2013;56:68-73. [Crossref] [PubMed]
- Larsson E, Vishnevskaya L, Kalin B, et al. High frequency of thoracic aneurysms in patients with abdominal aortic aneurysms. Ann Surg 2011;253:180-4. [Crossref] [PubMed]
- Hultgren R, Larsson E, Wahlgren CM, et al. Female and elderly abdominal aortic aneurysm patients more commonly have concurrent thoracic aortic aneurysm. Ann Vasc Surg 2012;26:918-23. [Crossref] [PubMed]
- Chaer RA, Vasoncelos R, Marone LK, et al. Synchronous and metachronous thoracic aneurysms in patients with abdominal aortic aneurysms. J Vasc Surg 2012;56:1261-5. [Crossref] [PubMed]
- Yaghoubian A, de Virgilio C, White RA, et al. Increased incidence of renal cysts in patients with abdominal aortic aneurysms: a common pathogenesis? Ann Vasc Surg 2006;20:787-91. [Crossref] [PubMed]
- Kim EK, Choi ER, Song BG, et al. Presence of simple renal cysts is associated with increased risk of aortic dissection: a common manifestation of connective tissue degeneration? Heart 2011;97:55-9. [Crossref] [PubMed]
- Chow K, Pyeritz RE, Litt HI. Abdominal visceral findings in patients with Marfan syndrome. Genet Med 2007;9:208-12. [Crossref] [PubMed]
- Ziganshin BA, Theodoropoulos P, Salloum MN, et al. Simple Renal Cysts as Markers of Thoracic Aortic Disease. J Am Heart Assoc 2016;5:e002248. [Crossref] [PubMed]
- Harada H, Furuya M, Ishikura H, et al. Expression of matrix metalloproteinase in the fluids of renal cystic lesions. J Urol 2002;168:19-22. [Crossref] [PubMed]
- Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol 2002;39:1890-900. [Crossref] [PubMed]
- Friedman T, Mani A, Elefteriades JA. Bicuspid aortic valve: clinical approach and scientific review of a common clinical entity. Expert Rev Cardiovasc Ther 2008;6:235-48. [Crossref] [PubMed]
- Verma S, Siu SC. Aortic dilatation in patients with bicuspid aortic valve. N Engl J Med 2014;370:1920-9. [Crossref] [PubMed]
- Davies RR, Kaple RK, Mandapati D, et al. Natural history of ascending aortic aneurysms in the setting of an unreplaced bicuspid aortic valve. Ann Thorac Surg 2007;83:1338-44. [Crossref] [PubMed]
- Michelena HI, Desjardins VA, Avierinos JF, et al. Natural history of asymptomatic patients with normally functioning or minimally dysfunctional bicuspid aortic valve in the community. Circulation 2008;117:2776-84. [Crossref] [PubMed]
- Elefteriades JA, Pomianowski P. Practical genetics of thoracic aortic aneurysm. Prog Cardiovasc Dis 2013;56:57-67. [Crossref] [PubMed]
- Pomianowski P, Elefteriades JA. The genetics and genomics of thoracic aortic disease. Ann Cardiothorac Surg 2013;2:271-9. [PubMed]
- Coady MA, Davies RR, Roberts M, et al. Familial patterns of thoracic aortic aneurysms. Arch Surg 1999;134:361-7. [Crossref] [PubMed]
- Biddinger A, Rocklin M, Coselli J, et al. Familial thoracic aortic dilatations and dissections: a case control study. J Vasc Surg 1997;25:506-11. [Crossref] [PubMed]
- Albornoz G, Coady MA, Roberts M, et al. Familial thoracic aortic aneurysms and dissections--incidence, modes of inheritance, and phenotypic patterns. Ann Thorac Surg 2006;82:1400-5. [Crossref] [PubMed]
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: executive summary. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Catheter Cardiovasc Interv 2010;76:E43-86. [Crossref] [PubMed]
- Erbel R, Aboyans V, Boileau C, et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2873-926. [Crossref] [PubMed]
- Ziganshin BA, Bailey AE, Coons C, et al. Routine Genetic Testing for Thoracic Aortic Aneurysm and Dissection in a Clinical Setting. Ann Thorac Surg 2015;100:1604-11. [Crossref] [PubMed]
- Falk RH. Images in clinical medicine. The "thumb sign" in Marfan's syndrome. N Engl J Med 1995;333:430. [Crossref] [PubMed]
- Loeys BL, Dietz HC, Braverman AC, et al. The revised Ghent nosology for the Marfan syndrome. J Med Genet 2010;47:476-85. [Crossref] [PubMed]
- Sato O, Takagi A, Miyata T, et al. Aortic aneurysms in patients with autoimmune disorders treated with corticosteroids. Eur J Vasc Endovasc Surg 1995;10:366-9. [Crossref] [PubMed]
- Slobodin G, Naschitz JE, Zuckerman E, et al. Aortic involvement in rheumatic diseases. Clin Exp Rheumatol 2006;24:S41-47. [PubMed]
- Robson JC, Kiran A, Maskell J, et al. The relative risk of aortic aneurysm in patients with giant cell arteritis compared with the general population of the UK. Ann Rheum Dis 2015;74:129-35. [Crossref] [PubMed]
- Evans JM, O'Fallon WM, Hunder GG. Increased incidence of aortic aneurysm and dissection in giant cell (temporal) arteritis. A population-based study. Ann Intern Med 1995;122:502-7. [Crossref] [PubMed]
- Mackie SL, Hensor EM, Morgan AW, et al. Should I send my patient with previous giant cell arteritis for imaging of the thoracic aorta? A systematic literature review and meta-analysis. Ann Rheum Dis 2014;73:143-8. [Crossref] [PubMed]
- Olsson C, Eriksson P, Franco-Cereceda A. Association between thoracic aortic disease and inguinal hernia. J Am Heart Assoc 2014.3. [PubMed]
- Fukunaga N, Yuzaki M, Nasu M, et al. Dissecting aneurysm in a patient with autosomal dominant polycystic kidney disease. Ann Thorac Cardiovasc Surg 2012;18:375-8. [Crossref] [PubMed]
- Lee CC, Chang WT, Fang CC, et al. Sudden death caused by dissecting thoracic aortic aneurysm in a patient with autosomal dominant polycystic kidney disease. Resuscitation 2004;63:93-6. [Crossref] [PubMed]
- Menon A, Sachithanandan A, Singh H, et al. Simultaneous aortic valve and arch replacement with bilateral nephrectomy for massive polycystic kidney disease, aortic regurgitation and dissecting aneurysm. Ann Thorac Surg 2011;91:919-20. [Crossref] [PubMed]
- Obuobie K, Smith J, Evans LM, et al. Increased central arterial stiffness in hypothyroidism. J Clin Endocrinol Metab 2002;87:4662-6. [Crossref] [PubMed]
- Dagre AG, Lekakis JP, Papaioannou TG, et al. Arterial stiffness is increased in subjects with hypothyroidism. Int J Cardiol 2005;103:1-6. [Crossref] [PubMed]
- Savage C, Deanfield JE, Jung RT. Aortic aneurysm in a patient with long-standing hypothyroidism. Postgrad Med J 1982;58:706-7. [Crossref] [PubMed]
- Rosenmann E, Yarom R. Dissecting aneurysm of the aorta and hypothyroidism. Isr J Med Sci 1994;30:510-3. [PubMed]
- van Bogerijen GH, Tolenaar JL, Grassi V, et al. Biomarkers in TAA-the Holy Grail. Prog Cardiovasc Dis 2013;56:109-15. [Crossref] [PubMed]
- Trimarchi S, Sangiorgi G, Sang X, et al. In search of blood tests for thoracic aortic diseases. Ann Thorac Surg 2010;90:1735-42. [Crossref] [PubMed]
- Wang Y, Barbacioru CC, Shiffman D, et al. Gene expression signature in peripheral blood detects thoracic aortic aneurysm. PLoS One 2007;2:e1050. [Crossref] [PubMed]





