Is the outcome in acute aortic dissection type A influenced by of femoral versus central cannulation?
Introduction
Acute aortic dissection type A (AADA) is a life threatening medical condition with still high mortality and morbidity requiring emergent surgical therapy (1-3). While without operation outcome is inacceptable (4,5), the use of extracorporeal circulation with hypothermic circulatory arrest is the gold-standard (6).
Replacement of the ascending aorta with resection of the entry site with an open distal ascending, hemiarch or total arch replacement, depending on the intima tears, is the standard approach. For the open distal anastomosis a period of hypothermic circulatory arrest often with the use of neuroprotective strategies like selective cerebral perfusion is needed (7). Survival of AADA patients is strongly dependent on an appropriate operative strategy, but the major influencing factors on mortality still remain uncertain. Extracorporeal circulation is needed in the therapy of AADA and the cannulation techniques might influence the outcome of the patients. Central cannulation in the ascending aorta has the advantage of antegrade body perfusion, but the risk of cannulating the false lumen. This might lead to progression of the dissection, malperfusion or aortic rupture. Therefore, cannulation of the subclavian artery—either directly or with an end-to-side prosthesis—is an alternative and adequate option in AADA patients. This is often termed as central cannulation, too, despite a short retrograde flow in this vessel. On the other hand, femoral artery cannulation is the easiest and fastest accessible site, but leads to retrograde flow in the descending and ascending aorta and might have an impact on plaque rupture and cerebral embolism. In addition, malperfusion might occur with retrograde flow (8). The primary objective of the present study is to analyze outcome parameters in a single-center series of AADA repair with different cannulation techniques for initial bypass.
Methods
Patients
All patients with diagnosed AADA operated between January 1, 2003 and December 31, 2015 were divided into two groups by the modus of initial arterial cannulation for extracorporeal circulation. Either central cannulation through the ascending aorta, right subclavian/axillary artery or right carotid artery (central cannulation group) or peripheral cannulation through the right or left femoral artery. No patient was excluded from the study. AADA was defined as diagnosed by echocardiography or computer tomography within of less than 12 hours before operation. The institutional review board approved this retrospective analysis; additional patient consent was not required.
Statistical analysis
All data were sampled retrospectively from the clinical data sheets. A χ2-test was used to compare categorical variables; a Student t-test was used to compare continuous variables. A P value of less than 0.05 was considered statistically significant. For mortality risk factor distribution a univariate analysis was performed. All significant parameters from the univariate analysis were included in a multivariate logistic regression. IBM SPSS Statistics Version 20 (IBM Germany, Ehningen) was used for all statistical analysis.
Surgical technique
A median sternotomy was performed in all patients. Aortic cannulation was performed after sternotomy. Subclavian/axillary, carotid or femoral cannulation was performed either before sternotomy or thereafter depending on the clinical status of the patient. Direct aortic cannulation was performed after heparinization in Seldinger technique guided by transesophageal echocardiography. If the Seldinger wire was in the true lumen of the aorta a ring reinforced aortic cannula was inserted (OptiSite, Edward Lifesciences Corporation Irvine, CA, USA or EOPA, Medtronic, Minneapolis, MN, USA) and fixed with tourniquets. Subclavian/axillary or carotid cannulation was performed through an extra incision above the vessel and direct cannulation in Seldinger technique or with prior implantation of an 8 mm Dacron graft onto the vessel after heparinization. Femoral cannulation was always performed with an open approach and direct cannulation in Seldinger technique (Fem-Flex, Edward Lifesciences Corporation Irvine, CA, USA). In the femoral cannulation group the arterial cannula was switched to central for re-starting of the extracorporeal circulation after circulatory arrest.
The criteria in selection of the cannulation site was depending on the experience of the surgeon and the experience of the anesthesiologist in preforming transesophageal echocardiography in combination with the hemodynamic state of the patients and the extent of the dissection and the dissection membrane.
The body temperature was monitored with bladder or rectum measurement and esophagus temperature.
Cerebral perfusion during circulatory arrest was performed either unilateral or bilateral depending on the oxygen consumption in the brain diagnosed by Near-Infrared Spectrograph (NIRS), which was routinely performed in all patients. Retrograde cerebral perfusion was not routinely performed.
The ascending aorta was replaced with a woven Dacron graft, which was curved if the Hemi-Arch or arch was involved, too. If the aortic valve or aortic root was involved root replacement was made either with a Bentall-de Bono procedure or a valve sparing repair according to David or Yacoub. A complete arch resection with an elephant trunk was performed in the conventional technique until 2013 and with the frozen Elephant technique since 2014.
Results
Two hundred and thirty five consecutive patients were diagnosed with AADA by contrast-enhanced computer tomography or echocardiography and underwent emergent surgery.
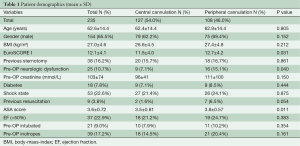
The main preoperative patient demographics are seen in Table 1. There were no significant differences in the preoperative demographic parameters, like age, gender, body-mass-index (BMI), previous sternotomy, diabetes, shock state and creatinine. In addition, the proportion of patients with low ejection fraction, preoperative intubation and inotrope dependence before the operation was similar. Significant differences were observed between the central and femoral cannulation group in EuroSCORE I (11.5±4.0 vs. 12.7±4.2, P=0.031), preoperative resuscitation (1.6% vs. 6.5%, P=0.040), preoperative neurologic dysfunction (7.1% vs. 15.1%, P=0.40) and ASA (American Society of Anesthesiologist) Physical Status Classification (3.5±0.81 vs. 3.8±0.57, P=0.011). 42.4% of the patients in the central cannulation group were classified in ASA score 2 to 3 (patient with mild or severe systemic disease) and 57.6% in the ASA score 4 to 5 (patient with severe systemic disease that is a constant threat to life or moribund patient who is not expected to survive without the operation). In the peripheral cannulation group 25.7% were in ASA score 2 to 3 and 74.9% in ASA score 4 to 5 (P=0.009).
Full table
Central cannulation was performed in in 127 patients (54%); via the subclavian/axillary artery in 104 patients (81.9%), directly via the ascending aorta in 22 patients (17.3%) and via the right carotid artery in one patient (0.8%). Extracorporeal circulation was started with femoral cannulation in 108 patients (46%). Venous drainage was performed either through the femoral vein (n=24, 10.2%) or a double-stage cannula in the right atrium (n=209, 88.9%). Bicaval venous cannulation was performed in 2 patients (0.9%).
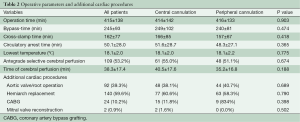
After starting extracorporeal circulation the body temperature was cooled to around 18 °C (18.1±2.0 vs. 18.1±2.2 °C, P=0.775). During hypothermic circulatory arrest direct cerebral perfusion was used in 55.0% with central cannulation and in 51.1% with femoral cannulation. Since 2013 the temperature management changed to higher temperatures of 24–26 °C with always antegrade cerebral perfusion. Concomitant cardiac procedures were coronary artery bypass grafting in 10.2% (n=24) and mitral valve reconstruction in 0.9% (n=2). An additional procedure on the aortic valve or aortic root was performed in 92 (39.3%) cases (Table 2).
Full table
Operation-, bypass- and X-clamp times were similar in both cannulation groups. In addition, the time of circulatory arrest and the time of cerebral perfusion were comparable.
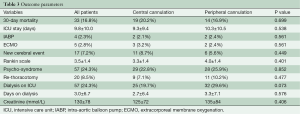
Table 3 shows the outcome parameter. 30-day-mortality (20.2% vs. 16.9%, P=0.699) and stay on the intensive care unit (ICU) (9.3±9.4 vs. 10.3±10.5, P=0.538) were similar in the central and peripheral cannulation group as was use of the intra-aortic balloon pump (2.1% vs. 2.4%, P=0.538) and need of extracorporeal membrane oxygenation (3.2% vs. 2.4%, P=0.561) postoperatively. The mean hospital length of stay was 17.2±18.1 days in the central cannulation and 19.1±16.4 days in the peripheral cannulation group (P=0.423).
Full table
A cerebral event or cerebral infarction in the computer tomographic scan was evident in 8.7% versus 5.6% (central vs. peripheral cannulation, P=0.449). The Rankin Scale of the patient with cerebral infarction was comparable between the cannulation groups (3.3±1.4 vs. 4.0±1.4, P=0.401). In addition, the development of a postoperative psycho-syndrome was similar with almost one fourth of all patients after operation. The need for a rethoracotomy was comparable between both cannulation groups (7.1% vs. 10.2%, P=0.477).
The amount of blood transfusion was 5.8±6.7 units and 7.2±11 units (P=0.246) in the central and peripheral cannulation groups. Thirty five (27.6%) of the central cannulation group and 25 (23.1%) of the peripheral cannulation group received no blood during hospital stay. The fresh frozen plasma transfusion was 3.2±5.0 units and 4.3±8.0 units (P=0.209). Seventy (55.1%) of the central cannulation group and 55 (50.9%) of the peripheral cannulation group received no fresh frozen plasma during hospital stay. Postoperative renal failure with the need for dialysis was borderline significant higher in the femoral artery group (38.6% vs. 26.6%, P=0.07).
Reintubation needed 17.3% (22 pts.) in the central cannulation and 19.4% (21 pts.) in the peripheral cannulation group (P=0.860). A tracheotomy was performed in 11.8% (15 pts.) in the central cannulation and in 12.0% (13 pts.) in the peripheral cannulation group (P=1.000).
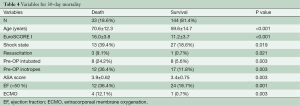
Table 4 shows the preoperative variables with significant impact on the 30-day-mortality. Only the variables with significance are plotted. Patients with a risk of 30-day-mortality are significant older and with a higher EuroSCORE I and ASA-Classification. Additional parameters were preoperative shock state, need for resuscitation, inotrope dependency and intubation. However, in the multivariate analysis support only EuroSCORE I above 13 was a significant risk factor for 30-day-mortality in our patients (Table 5).
Full table
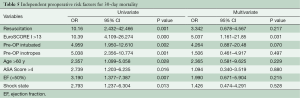
Full table
Discussion
Mortality with acute aortic dissection type A remains high with an average 30-day-mortality of around 17%, which progressively increase to 25% in octogenarians (2). Despite modern surgical techniques mortality remained constantly high over the last decade (1). In our single-center study we report a 30-day-mortality of 16.8% in 235 patients, which confirms multi-center registry studies with 30-day mortalities between 16.9% and 22% (1,9). Our cannulation strategy for initial bypass had no impact on mortality. While some studies show a positive effect with central cannulation (10) other studies could not demonstrate any benefit (1,11-13). In a meta-analysis from Patris et al. femoral cannulation had a negative outcome, but was used mainly in critically ill patients in hemodynamic collapse when institution of cardiopulmonary bypass was required rapidly (14). Our data show, that femoral cannulation was performed more often in a sicker patient group measured by the ASA classification. However, the ASA classification is still a subjective classification dependent from the judgment of the anesthesiologist. Nevertheless, higher EuroSCORE I, previous resuscitation and preoperative neurologic dysfunction were significant more often in femoral cannulation group. The reason might be a faster access to the cannulation site, compared to the subclavian artery, in the need for a fast sternotomy due the pericardial effusion/tamponade and hemodynamic instability. Despite this sicker and hemodynamic instable patient group outcome parameter were not adversely affected by femoral cannulation. In the study from Etz et al. the group with femoral artery cannulation showed significant shorter operation and circulatory arrest times. However, outcome parameters, especially postoperative stroke (18% vs. 15%) and early death (21 vs. 20%, peripheral vs. central cannulation) were comparable (11). In the meta-analysis from Tiwari et al. just axillary artery cannulation was superior regarding outcome but not central cannulation in total (15). In addition, the study from Kamiya et al. could only show a borderline significance with aortic in contrast to femoral cannulation regarding 30-day-mortality, despite higher ASA classification in the femoral cannulation group (13). The large German Registry for acute aortic dissection (GERAADA) with 2,137 patients could not show any significant impact of the cannulation site on any outcome parameter (1).
Preoperative neurologic symptoms were presented in around 7% and postoperative neurologic symptoms in up to 20% of the patients (1). In our study, we could not find any differences in neurologic symptoms regarding the perfusion strategy. In a meta-analysis of nine acute type A dissection studies Ren et al. could show a significant benefit in short-term and postoperative neurology in patients with axillary cannulation in contrast to femoral artery cannulation (12). As a limitation of this study, it is not known if selective cerebral perfusion was performed during circulatory arrest and which was the lowest body temperature. In our study, we used deep circulatory arrest with an average temperature of 18.1 °C and additional selective antegrade cerebral perfusion in 53% of all patients. The time of cerebral perfusion was in average 76.8% of the time of circulatory arrest with no differences between both perfusion groups. However, the use of additional selective cerebral perfusion had no impact in postoperative mortality or neurology in our study at an average temperature of 18 °C. With adequate cerebral perfusion and cerebral monitoring a moderate hypothermic arrest with temperatures between 24 and 28 °C are acceptable. In the setting of antegrade cerebral perfusion, deep hypothermia does not provide any additional benefit (16). Conzelmann et al. could similar show that neurological complications does not seem to be affected by choice of the arterial cannulation site (17).
Similar to the multi-center study from Rylski et al. age was not a predictor for worse neurologic outcome but for mortality (2). Our 30-day-mortality was 7% in the age group below 50 years, 15.3% in the group between 50 and 70 and 28% above 70 years of age (P=0.023).
Univariate and multivariate logistic regression analysis was performed to characterize risk factors for 30-day-mortality. Age was a significant factor for worse 30-day-mortality as was worse clinical appearance before the operation (EuroSCORE I, shock, reanimation, intubation, inotropic support). In addition, only one patient with postoperative ECMO support after operation of acute type A aortic dissection survived. However, in the multivariate analysis the only preoperative significant independent risk factor for 30-day-mortality was EuroSCORE I above 13.
Limitations
This study has some limitations, which needed to be addressed. First, this is a retrospective data analysis in a very heterogeneous patient population. In addition, the choice of cannulating a central or peripheral vessel was mainly driven by the experience of the surgeon rather than objective parameters.
Conclusions
Our single-center study with 235 patients operated on AADA revealed that the cannulation technique (femoral or central vessels) did not have any impact on postoperative neurology or 30-day-mortaliy or any other postoperative outcome parameter. This might be triggered by the constantly use of circulatory arrest at a profound hypothermic state with a body temperature around 18 °C. This low temperature did not affect the average operation time or the need for re-thoracotomy for bleeding compared to the literature. Mortality in the early 30 days after the operation was depending on the hemodynamic state and the overall clinical status measured by the EuroScore I and ASA classification at the time of presenting in the operation room. Higher age was a risk factor for mortality but not postoperative neurology.
We conclude, that femoral artery cannulation is a safe and easy accessible technique with similar outcome in AADA patients, which is still useful especially in hemodynamic instable patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The institutional review board approved this retrospective analysis; additional patient consent was not required.
References
- Conzelmann LO, Weigang E, Mehlhorn U, et al. Mortality in patients with acute aortic dissection type A: analysis of pre- and intraoperative risk factors from the German Registry for Acute Aortic Dissection Type A (GERAADA). Eur J Cardiothorac Surg 2016;49:e44-52. [Crossref] [PubMed]
- Rylski B, Hoffmann I, Beyersdorf F, et al. Acute aortic dissection type A: age-related management and outcomes reported in the German Registry for Acute Aortic Dissection Type A (GERAADA) of over 2000 patients. Ann Surg 2014;259:598-604. [Crossref] [PubMed]
- Rylski B, Suedkamp M, Beyersdorf F, et al. Outcome after surgery for acute aortic dissection type A in patients over 70 years: data analysis from the German Registry for Acute Aortic Dissection Type A (GERAADA). Eur J Cardiothorac Surg 2011;40:435-40. [PubMed]
- Matsui M, Toshikazu G, Yahagi T, et al. Prognosis of type A acute aortic dissection treated conservatively. Kyobu Geka 2010;63:89-94; discussion 94-7. [PubMed]
- Ando T, Kobayashi T, Endo H, et al. Surgical treatment or conservative therapy for stanford type a acute aortic dissection with a thrombosed false lumen. Ann Vasc Dis 2012;5:428-34. [Crossref] [PubMed]
- Krüger T, Conzelmann LO, Bonser RS, et al. Acute aortic dissection type A. Br J Surg 2012;99:1331-44. [Crossref] [PubMed]
- Easo J, Weigang E, Hölzl PP, et al. Influence of operative strategy for the aortic arch in DeBakey type I aortic dissection - analysis of the German Registry for Acute Aortic Dissection type A (GERAADA). Ann Cardiothorac Surg 2013;2:175-80. [PubMed]
- Shimokawa T, Takanashi S, Ozawa N, et al. Management of intraoperative malperfusion syndrome using femoral artery cannulation for repair of acute type A aortic dissection. Ann Thorac Surg 2008;85:1619-24. [Crossref] [PubMed]
- Pape LA, Awais M, Woznicki EM, et al. Presentation, Diagnosis, and Outcomes of Acute Aortic Dissection: 17-Year Trends From the International Registry of Acute Aortic Dissection. J Am Coll Cardiol 2015;66:350-8. [Crossref] [PubMed]
- Schurr UP, Emmert MY, Berdajs D, et al. Subclavian artery cannulation provides superior outcomes in patients with acute type-A dissection: long-term results of 290 consecutive patients. Swiss Med Wkly 2013;143:w13858. [PubMed]
- Etz CD, von Aspern K, da Rocha E, Silva J, et al. Impact of perfusion strategy on outcome after repair for acute type a aortic dissection. Ann Thorac Surg 2014;97:78-85. [Crossref] [PubMed]
- Ren Z, Wang Z, Hu R, et al. Which cannulation (axillary cannulation or femoral cannulation) is better for acute type A aortic dissection repair? A meta-analysis of nine clinical studies. Eur J Cardiothorac Surg 2015;47:408-15. [Crossref] [PubMed]
- Kamiya H, Kallenbach K, Halmer D, et al. Comparison of ascending aorta versus femoral artery cannulation for acute aortic dissection type A. Circulation 2009;120:S282-6. [Crossref] [PubMed]
- Patris V, Toufektzian L, Field M, et al. Is axillary superior to femoral artery cannulation for acute type A aortic dissection surgery? Interact Cardiovasc Thorac Surg 2015;21:515-20. [Crossref] [PubMed]
- Tiwari KK, Murzi M, Bevilacqua S, et al. Which cannulation (ascending aortic cannulation or peripheral arterial cannulation) is better for acute type A aortic dissection surgery? Interact Cardiovasc Thorac Surg 2010;10:797-802. [Crossref] [PubMed]
- Leshnower BG, Thourani VH, Halkos ME, et al. Moderate Versus Deep Hypothermia With Unilateral Selective Antegrade Cerebral Perfusion for Acute Type A Dissection. Ann Thorac Surg 2015;100:1563-8; discussion 1568-9. [Crossref] [PubMed]
- Conzelmann LO, Hoffmann I, Blettner M, et al. Analysis of risk factors for neurological dysfunction in patients with acute aortic dissection type A: data from the German Registry for Acute Aortic Dissection type A (GERAADA). Eur J Cardiothorac Surg 2012;42:557-65. [Crossref] [PubMed]





