Differential aspects of ascending thoracic aortic dissection and its treatment: the North American experience
Introduction
There is no consensus regarding the optimal surgical approach for patients with the life-threatening condition of acute type A aortic dissection. Tube graft replacement of the ascending aorta and hemiarch replacement is the chief simple approach, but various aortic surgery programs are evaluating more aggressive approaches, including total arch replacement with or without the use of stent graft in the descending thoracic aorta [i.e., frozen elephant trunk (FET)].
Differential aspects of the disease
According to the Stanford classification, type A aortic dissection involves the ascending aorta, the aortic arch, or both, and possibly the descending aorta; the tear originates in the ascending aorta, the aortic arch, or, more rarely, in the descending aorta. In the DeBakey classification, type I and type II acute aortic dissections involve the ascending aorta from the aortic root through the origin of the innominate artery (type II) or through the entire descending and abdominal aorta (type I). DeBakey types I and II are both included in the Stanford type A. Neither classification applies to dissection limited to the aortic arch.
Acute aortic syndrome consists of classic acute aortic dissection, intramural hematoma, and penetrating atherosclerotic ulcer. In classic acute aortic dissection, the tear originates in the intima and allows blood to travel between the intima and the diseased underlying media, thus creating a false lumen. Pressurized blood can cause the false lumen to propagate proximally and distally, potentially resulting in malperfusion, aortic regurgitation, stroke, cardiac tamponade, rupture in the mediastinum or pleural space, and death.
Intramural hematoma, another variant of aortic dissection, results from rupture of the vasa vasorum without an intimal tear (1); there is no communication with the aortic lumen unless the hematoma evolves into a dissection, which happens in one third of cases. The majority of intramural hematomas occur in the descending aorta. Patients with intramural hematoma are usually older and more hypertensive than patients with classic acute aortic dissection (1,2). The hematoma can be focal or can extend proximally and distally.
The penetrating atheromatous ulceration of the aortic wall was first described by Shennan (3) and further characterized by Stanson et al. (4) as disrupting and tunneling through the intima into the media. Ulceration can cause localized intramedial dissection, which is limited by severe atherosclerosis and which causes a variable amount of hematoma (1). This localized dissection can break into the adventitia, causing pseudoaneurysm or rupture. Most cases occur in elderly patients with significant underlying atherosclerosis and frequently involve the descending thoracic aorta (1,5). There is no branch-vessel occlusion, which can occur in classic acute aortic dissection.
Iatrogenic acute type A aortic dissection can be the result of a cardiac operation or the manipulation of catheters and wires during thoracic endovascular aortic repair (TEVAR) of the descending aorta (6-8). Common dissection sites after a cardiac operation are the aortic root vent, the aortic cannulation site and the site of the proximal anastomoses (7).
Diagnosis
Chest pain is the presenting symptom of acute aortic syndromes involving the ascending aorta. Patients with classic acute ascending aortic dissection may have symptoms of congestive heart failure due to dissection-related aortic valve insufficiency and cardiac ischemia due to dissection-related coronary occlusion.
Classic acute proximal aortic dissection (i.e., type I or type A aortic dissection) can cause central neurologic symptoms due to dissection of the head vessels or peripheral neurologic symptoms (lower-extremity paralysis or paraparesis) due to distal aortic vessel dissection and occlusion. Mesenteric ischemia and renal malperfusion due to visceral branch vessel occlusion are additional signs of classic aortic dissection, causing abdominal or flank pain and elevated blood creatinine levels. Pulse inequality can also occur.
The majority of patients with type A aortic dissection are male, Caucasian, and in their early 60s (9), with the exception of patients with genetically triggered thoracic aortic disease who are usually less than 50 years of age. In acute cases, the noninvasive modality of choice in a stable patient with type A distribution dissection is gated, contrast-enhanced spiral computed tomographic angiography (CTA), which has a reported diagnostic sensitivity of 93% (9). Electrocardiographic gating is mandatory in order to avoid motion artifacts that could mimic the ‘curtain-like’ flap that is typically associated with classic acute proximal ascending aortic dissection. This flap traverses the ascending aortic lumen and is sometimes accompanied by bloody pericardial or pleural effusion.
Other differential diagnoses for acute aortic syndrome that can be diagnosed by CTA include acute intramural hematoma and aortic ulceration. In the noncontrast sequence of the CTA, intramural hematoma can appear as a “rind” or crescentic hyperattenuation in the aortic wall (with Hounsfield units measured between 40–60 s) that extends partially or circumferentially along the aortic wall, with or without intimal displacement of calcification. In the contrast-enhanced CTA, the intramural hematoma will not enhance, and no dissection flap should be seen (10). In acute aortic ulceration, there will be focal contrast collection or crater projecting beyond the aortic lumen on CT, often in association with atheroma, thrombus, or both (11).
Magnetic resonance imaging or angiography is rarely used as the initial diagnostic modality, and the use of transesophageal echocardiography for this purpose has decreased over time (9), most likely because of increased use of spiral chest CT.
Treatment
Emergent or urgent surgical intervention is the standard of care for classic acute type A aortic dissection unless prohibitive comorbidities concomitant with extremely advanced age preclude such repair (12). The primary goal of the operation is to prevent immediate death; the secondary goal is to promote long-term survival and avoid further reoperation on the distal aorta. Major aortic surgery centers have described various extents of surgical repair with hemiarch (i.e., proximal arch) or total arch replacement and different adjuncts for brain protection [antegrade or retrograde cerebral perfusion (RCP)] (13-22). Most North American centers (13-19,23) tend to favor ascending aorta and hemiarch replacement, with more extensive arch replacement in cases in which the arch is dilated more than 4.5–5 cm or the tear involves the aortic arch. Reportedly, both deep and moderate levels of hypothermia during the period of circulatory arrest have been used successfully (14-17,22). Different cannulation strategies have been advocated (19,21,23-27). Early application of the cross-clamp during the cooling period has been reported (7,23), as has a clampless technique (15-19). Operative details from North American cardiac surgery centers are shown in Table 1.
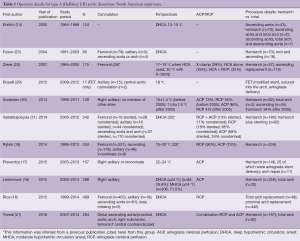
Full table
Overall operative mortality reported by North American centers varies from 5% to 17% (13-23) and may improve after protocol-based management is implemented and a thoracic aortic team is assembled (30,34). Mortality associated specifically with iatrogenic acute type A dissection has been recently reported as 27% after open surgical repair and up to 33–50% after TEVAR (6-8). These high mortality rates may be explained by the superimposed, complex, or extensive dissection that characterizes iatrogenic cases; advanced patient age; or the need to revise the original cardiac procedure in addition to repairing the iatrogenic ascending dissection. The surgical mortality rate for acute type A aortic dissection in patients with hemodynamic instability varies from 25.3% to 47% (34,35). Outcome data from North American cardiac surgery centers are shown in Table 2.
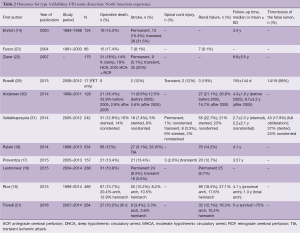
Full table
Because of recent advances in TEVAR and evidence that it results in aortic remodeling and future false-lumen thrombosis in patients with aortic dissection in the descending thoracic aorta (36), certain aortic surgery centers have advocated antegrade or retrograde stent delivery in the descending thoracic aorta with simultaneous hemiarch or arch surgery for acute type I aortic dissection (29,31,37). This approach is thought to promote remodeling of the distal aorta and prevent chronic dissecting aneurysm formation that would necessitate additional distal aortic intervention. Reportedly, the additional stent delivery during the procedure is safe, resolves possible malperfusion, and promotes aortic remodeling (29,31,37). Spinal cord ischemia remains a concern with simultaneous antegrade or retrograde stent delivery; thus, stent length is usually 15 cm or less.
Additional follow-up and long-term studies are needed to validate these findings. The reported 10-year survival rate after acute type A (DeBakey type I and type II) aortic dissection varies between 41% and 61% (18,19). In extremely rare circumstances, custom-made endografts and/or endografts approved for the descending aorta have been used to treat ascending aortic dissection, but experience is limited and anecdotal (38). There are no currently approved endografts for the ascending aorta, even though US trials to treat aneurysmal disease of the ascending aorta and proximal dissection are forthcoming.
Two prostheses are commercially available in Europe. Both consist of a vascular graft with a distal stent graft: the E-vita Open Plus prosthesis (JOTEC GmbH, Hechingen, Germany) and the Thoraflex hybrid prosthesis (Vascutek, Inchinnan, Scotland). These two available prostheses may be driving the trend toward total arch replacement in patients with type A aortic dissection (39,40). It is possible (but this is pure speculation) that when these prostheses become available in the United States, we will see a trend toward total arch replacement with FET in patients with type I aortic dissection (or type A aortic dissection extending into the descending thoracic aorta).
How we do it: steps of surgical repair
- Intraoperative transesophageal echocardiography is performed before sternotomy in all patients except those in extremis.
- Near-infrared spectroscopy probes are placed over the cranium to monitor cerebral perfusion by measuring regional cerebral oxygen saturation.
- In hemodynamically stable patients, arterial inflow is established via an 8-mm Dacron graft into the innominate artery if the dissection does not extend into it, or via the Dacron graft into the right axillary artery (24). If the patient is in extremis, femoral artery cannulation is another alternative (depending on the body habitus), as is direct aortic cannulation. In rupture cases, cardiopulmonary bypass (CPB) is occasionally initiated before sternotomy.
- A left ventricular vent is inserted in all cases, usually via the right superior pulmonary vein. A retrograde cardioplegia cannula is also inserted in all cases unless insertion is technically cumbersome.
- After CPB is initiated, cooling begins. To avoid further damage and prevent the dissection from propagating, we do not cross-clamp the aorta early in the cooling phase. We apply the cross-clamp early only when the heart becomes distended (despite the left ventricular vent) because of massive aortic insufficiency.
- For brain protection, we use antegrade cerebral perfusion (ACP) (usually unilaterally, but bilaterally when we anticipate that reconstruction will take more than 20–30 min) (17). Lately, it has been our default preference to use bilateral ACP because it is quick and easy. We use a moderate level of hypothermia (approximately 24 °C nasopharyngeal temperature). Because of ACP, our practice has shifted to warmer temperatures. We do not use RCP.
- Once the target temperature is reached, we ask the perfusionist to turn the flows down to 10–15 mL/kg/min. A Rumel tourniquet or a clamp is then applied to the innominate artery, and ACP is initiated. Our goal is to maintain the perfusion pressure at 50–70 mmHg. When the right axillary artery or the innominate artery is used for arterial inflow, the perfusion pressure of 50–70 mmHg is the right radial arterial line pressure.
- The false and true lumens are opened, and the aorta is inspected to identify the aortic tear. The thrombus between the true and false lumens is removed. A balloon-tip catheter is used for ACP in the left common carotid artery after the aorta is divided (17). Myocardial protection is achieved with retrograde and antegrade cardioplegia. The antegrade cardioplegia solution is administered directly into the coronary ostia.
- It is our approach to proceed with hemiarch replacement in most cases. The layers of the true and false lumens are approximated circumferentially with a few 6-0 prolene stitches, and Bioglue (Cryolife, Inc., Kennesaw, GA, USA) is applied between the layers. For the distal anastomosis, we use a Dacron Gelweave graft (Vascutek Ltd, Renfrewshire, Scotland, UK) and two layers of running 3-0 or 4-0 (or occasionally 5-0) polypropylene sutures. Depending on the strength of the tissues, a row of pledgeted 4-0 polypropylene sutures is placed circumferentially in addition to or instead of the second running row. When the aortic ach is dilated or the tear is in the transverse arch and cannot be repaired otherwise, we proceed with total arch replacement. In cases of acute type A aortic dissection, we do not replace the total arch unless it is absolutely necessary (37). Instead, we prefer to perform antegrade stent graft delivery in the descending thoracic aorta (acute type I aortic dissection) over a stiff wire as previously described (37) and then to reconstruct the distal aortic anastomosis. After stent graft deployment, the distal anastomosis is constructed by leaving only a small, V-shaped piece of native aortic tissue at the arch. We are more likely to use antegrade stent delivery in the descending thoracic aorta for patients with malperfusion due to acute type I aortic dissection or with a dilated distal aortic arch.
- After the distal open anastomosis is completed, we ask the perfusionist to restore full flow, and the Rumel tourniquet or clamp is removed from the innominate artery. A cross-clamp is applied to the Dacron graft, and full flow is initiated. The rewarming process is then begun.
- With regard to the aortic valve: We transect the aortic tissue approximately 5 mm above the sinotubular junction. The true and the false lumens are brought together with 6-0 prolene sutures. Depending on the structure and function of the aortic valve, we usually perform resuspension or commissuroplasty. In patients with a connective tissue disorder or annuloaortic ectasia, we replace the aortic root by using a modified Bentall procedure. If the sinuses are normal but the valve leaflets are destroyed, we replace the aortic valve. Often, a piece of Teflon is inserted into the false lumen at the area of the noncoronary sinus, and 6-0 or 5-0 prolene is used to approximate all of these layers. If the non-coronary sinus is enlarged but the other sinuses are normal, the aortic valve leaflets are not destroyed, and there is no need to replace the aortic root; we remove part of the aortic tissue of the noncoronary sinus close to the annulus. We do not usually perform aortic valve–sparing root replacement (AVSRR) in patients with acute type I aortic dissection. We reserve AVSRR for patients who are stable, with no signs of hemodynamic instability, or whose aortic dissection is mainly limited to the ascending aorta.
- After the proximal anastomosis and all associated procedures are completed, deairing follows, and the cross-clamp is removed.
- Once the nasopharyngeal temperature reaches 36.5 °C, we wean the patient from CPB, administer protamine, and establish hemostasis.
- After cross-clamp removal and during hemostasis, we avoid manipulating the aortic graft. In most cases, no additional repair stitches are needed.
Acknowledgements
Stephen N. Palmer, PhD, ELS, and Kelly Tucker, MS, contributed to the editing of the manuscript.
Footnote
Conflicts of Interest: Dr. Coselli participates in clinical research trials conducted by Glaxo Smith Kline, Edwards Lifesciences, and Bolton Medical; consults for, receives royalties and a departmental educational grant from, and participates in clinical trials for Vascutek Terumo; and consults and participates in clinical trials for Medtronic, Inc. and W. L. Gore & Associates. Dr. Preventza consults for Medtronic, Inc. and W. L. Gore & Associates, and she has received travel expenses from Cook Medical, Inc.
References
- Coady MA, Rizzo JA, Elefteriades JA. Pathologic variants of thoracic aortic dissections. Penetrating atherosclerotic ulcers and intramural hematomas. Cardiol Clin 1999;17:637-57. [Crossref] [PubMed]
- Evangelista A, Mukherjee D, Mehta RH, et al. Acute intramural hematoma of the aorta: a mystery in evolution. Circulation 2005;111:1063-70. [Crossref] [PubMed]
- Shennan T. Dissecting aneurysms. Special report series no. 193. Medical Research Council 1934.
- Stanson AW, Kazmier FJ, Hollier LH, et al. Penetrating atherosclerotic ulcers of the thoracic aorta: natural history and clinicopathologic correlations. Ann Vasc Surg 1986;1:15-23. [Crossref] [PubMed]
- Cooke JP, Kazmier FJ, Orszulak TA. The penetrating aortic ulcer: pathologic manifestations, diagnosis, and management. Mayo Clin Proc 1988;63:718-25. [Crossref] [PubMed]
- Preventza O, Garcia A, Moeller K, et al. Retrograde ascending aortic dissection after thoracic endovascular aortic repair for distal aortic dissection or with zone 0 landing: association, risk factors, and true incidence. Ann Thorac Surg 2015;100:509-15. [Crossref] [PubMed]
- Stamou SC, Kouchoukos NT, Hagberg RC, et al. Differences in clinical characteristics, management, and outcomes of intraoperative versus spontaneous acute type A aortic dissection. Ann Thorac Surg 2013;95:41-5. [Crossref] [PubMed]
- Williams JB, Andersen ND, Bhattacharya SD, et al. Retrograde ascending aortic dissection as an early complication of thoracic endovascular aortic repair. J Vasc Surg 2012;55:1255-62. [Crossref] [PubMed]
- Pape LA, Awais M, Woznicki EM, et al. Presentation, diagnosis, and outcomes of acute aortic dissection: 17-year trends from the International Registry of Acute Aortic Dissection. J Am Coll Cardiol 2015;66:350-8. [Crossref] [PubMed]
- Chao CP, Walker TG, Kalva SP. Natural history and CT appearances of aortic intramural hematoma. Radiographics 2009;29:791-804. [Crossref] [PubMed]
- Scheske JA, Chung JH, Abbara S, et al. Computed tomography angiography of the thoracic aorta. Radiol Clin North Am 2016;54:13-33. [Crossref] [PubMed]
- Elefteriades JA. Editorial comment: acute type A aortic dissection: surgical intervention for all. Cardiol Clin 2010;28:333-4. [Crossref] [PubMed]
- Crawford ES, Svensson LG, Coselli JS, et al. Surgical treatment of aneurysm and/or dissection of the ascending aorta, transverse aortic arch, and ascending aorta and transverse aortic arch. Factors influencing survival in 717 patients. J Thorac Cardiovasc Surg 1989;98:659-73; discussion 73-4. [PubMed]
- Ehrlich MP, Ergin MA, McCullough JN, et al. Results of immediate surgical treatment of all acute type A dissections. Circulation 2000;102:III248-52. [Crossref] [PubMed]
- Lawton JS. Acute type A aortic dissection 101. J Thorac Cardiovasc Surg 2015;150:769-70. [Crossref] [PubMed]
- Leshnower BG, Thourani VH, Halkos ME, et al. Moderate versus deep hypothermia with unilateral selective antegrade cerebral perfusion for acute type A dissection. Ann Thorac Surg 2015;100:1563-8; discussion 8-9. [Crossref] [PubMed]
- Preventza O, Simpson KH, Cooley DA, et al. Unilateral versus bilateral cerebral perfusion for acute type A aortic dissection. Ann Thorac Surg 2015;99:80-7. [Crossref] [PubMed]
- Rice RD, Sandhu HK, Leake SS, et al. Is total arch replacement associated with worse outcomes during repair of acute type A aortic dissection? Ann Thorac Surg 2015;100:2159-65; discussion 2165-6. [Crossref] [PubMed]
- Rylski B, Milewski RK, Bavaria JE, et al. Long-term results of aggressive hemiarch replacement in 534 patients with type A aortic dissection. J Thorac Cardiovasc Surg 2014;148:2981-5. [Crossref] [PubMed]
- Svensson LG, Crawford ES, Hess KR, et al. Dissection of the aorta and dissecting aortic aneurysms. Improving early and long-term surgical results. Circulation 1990;82:IV24-38. [PubMed]
- Trivedi D, Navid F, Balzer JR, et al. Aggressive aortic arch and carotid replacement strategy for type A aortic dissection improves neurologic outcomes. Ann Thorac Surg 2016;101:896-903; discussion 903-5. [Crossref] [PubMed]
- Zierer A, Moon MR, Melby SJ, et al. Impact of perfusion strategy on neurologic recovery in acute type A aortic dissection. Ann Thorac Surg 2007;83:2122-8; discussion 8-9. [Crossref] [PubMed]
- Fusco DS, Shaw RK, Tranquilli M, et al. Femoral cannulation is safe for type A dissection repair. Ann Thorac Surg 2004;78:1285-9; discussion -9.
- Preventza O, Garcia A, Tuluca A, et al. Innominate artery cannulation for proximal aortic surgery: outcomes and neurological events in 263 patients. Eur J Cardiothorac Surg 2015;48:937-42; discussion 42. [Crossref] [PubMed]
- Strauch JT, Spielvogel D, Lauten A, et al. Axillary artery cannulation: routine use in ascending aorta and aortic arch replacement. Ann Thorac Surg 2004;78:103-8; discussion 103-8. [Crossref] [PubMed]
- Svensson LG, Blackstone EH, Rajeswaran J, et al. Does the arterial cannulation site for circulatory arrest influence stroke risk? Ann Thorac Surg 2004;78:1274-84; discussion 1274-84. [Crossref] [PubMed]
- Wong DR, Coselli JS, Palmero L, et al. Axillary artery cannulation in surgery for acute or subacute ascending aortic dissections. Ann Thorac Surg 2010;90:731-7. [Crossref] [PubMed]
- Moon MR, Sundt TM 3rd, Pasque MK, et al. Does the extent of proximal or distal resection influence outcome for type A dissections? Ann Thorac Surg 2001;71:1244-9; discussion 9-50. [Crossref] [PubMed]
- Roselli EE, Rafael A, Soltesz EG, et al. Simplified frozen elephant trunk repair for acute DeBakey type I dissection. J Thorac Cardiovasc Surg 2013;145:S197-201. [Crossref] [PubMed]
- Andersen ND, Ganapathi AM, Hanna JM, et al. Outcomes of acute type A dissection repair before and after implementation of a multidisciplinary thoracic aortic surgery program. J Am Coll Cardiol 2014;63:1796-803. [Crossref] [PubMed]
- Vallabhajosyula P, Szeto WY, Pulsipher A, et al. Antegrade thoracic stent grafting during repair of acute Debakey type I dissection promotes distal aortic remodeling and reduces late open distal reoperation rate. J Thorac Cardiovasc Surg 2014;147:942-8. [Crossref] [PubMed]
- Pochettino A, Brinkman WT, Moeller P, et al. Antegrade thoracic stent grafting during repair of acute DeBakey I dissection prevents development of thoracoabdominal aortic aneurysms. Ann Thorac Surg 2009;88:482-9; discussion 9-90. [Crossref] [PubMed]
- Bavaria JE, Pochettino A, Brinster DR, et al. New paradigms and improved results for the surgical treatment of acute type A dissection. Ann Surg 2001;234:336-42; discussion 42-3. [Crossref] [PubMed]
- Grau JB, Kuschner CE, Ferrari G, et al. Effects of a protocol-based management of type A aortic dissections. J Surg Res 2015;197:265-9. [Crossref] [PubMed]
- Conway BD, Stamou SC, Kouchoukos NT, et al. Effects of hemodynamic instability on early outcomes and late survival following repair of acute type A aortic dissection. Aorta (Stamford) 2014;2:22-7. [Crossref] [PubMed]
- Nienaber CA, Kische S, Rousseau H, et al. Endovascular repair of type B aortic dissection: long-term results of the randomized investigation of stent grafts in aortic dissection trial. Circ Cardiovasc Interv 2013;6:407-16. [Crossref] [PubMed]
- Preventza O, Cervera R, Cooley DA, et al. Acute type I aortic dissection: traditional versus hybrid repair with antegrade stent delivery to the descending thoracic aorta. J Thorac Cardiovasc Surg 2014;148:119-25. [Crossref] [PubMed]
- Berfield KK, Sweet MP, McCabe JM, et al. Endovascular repair for type A aortic dissection after transcatheter aortic valve replacement with a Medtronic CoreValve. Ann Thorac Surg 2015;100:1444-6. [Crossref] [PubMed]
- Di Bartolomeo R, Pantaleo A, Berretta P, et al. Frozen elephant trunk surgery in acute aortic dissection. J Thorac Cardiovasc Surg 2015;149:S105-9. [Crossref] [PubMed]
- Shrestha M, Fleissner F, Ius F, et al. Total aortic arch replacement with frozen elephant trunk in acute type A aortic dissections: are we pushing the limits too far? Eur J Cardiothorac Surg 2015;47:361-6; discussion 6. [Crossref] [PubMed]





