Degenerative mitral valve disease-contemporary surgical approaches and repair techniques
Introduction
Myxomatous degeneration of mitral valve apparatus affects 2–3% of the general population and it is the most common cause of severe mitral regurgitation in western countries (1). More than 90% of degenerative mitral valves are suitable for valve repair rather than replacement, with short and long term clinical outcomes being superior after repair (2). In addition to the clinical benefits, mitral valve repair is associated with an economic benefit in the form of reduced short and long term medical expenses (3,4). Patients who have undergone mitral valve repair have mitral function restored, have avoided the added expense of a prosthetic valve and are free from lifelong anti-coagulation requirement.
The 2014 American College of Cardiology/American Heart Association (ACC/AHA) guidelines for management of patients with valvular heart disease recommend early surgery, before signs of LV function deterioration, and that asymptomatic patients with severe mitral regurgitation should be offered surgery in Heart Valve Centers of Excellence if there is a high probability of successful repair and low mortality risk (5-8). Despite the well accepted data favoring mitral valve repair, repair rates are not uniformly distributed in the United States. The mean rate of repair is 69%, ranging from 35% in low volume hospitals, trending to 90% in tertiary centers with high case volumes (9,10). Hospital volume, primarily driven by individual surgeon volume, is associated with lower postoperative mortality rates reported for repair at 1.0% for high and 2.7% for low volume surgeons and for replacement at 3.6% for high and 7.4% for low volume surgeons (11). Thus, the discrepancy in mortality and repair rates is probably somewhat related to surgeons’ experience and comfort level with repair techniques. Given the increasing age of the US population and the concomitant expected rise in cardiovascular disease, we will likely see increasing numbers of degenerative mitral valve pathology in a more complex population (12). Increasing the overall rate of mitral valve repair will become more important as time goes on and providing a universally and uniformly accepted quality of repair should have beneficial medical, economic, and societal implications.
This article describes preoperative and intraoperative considerations in mitral valve repair as well as currently practiced repair approaches and techniques. The aim is to articulate our contemporary approach of mitral valve repair so that it may be used as a model by other institutions that may have low repair rates. Adoption of simple and reproducible mitral valve repair techniques is of paramount importance if we are to accomplish overall higher rates of mitral valve repair and optimal outcomes.
Patient selection and preoperative work-up
When considering the optimal surgical approach for a given patient, we primarily consider patient’s comorbidities, as most severe adverse outcomes are complications related to existing comorbidities. Additionally, the surgeon’s level of expertise and the increasing preference expressed by patients for minimally invasive procedures must be taken into account.
Our primary goal is to choose the approach that will yield a safe and effective operation, and our secondary goal is to achieve this through the smallest incision possible. To maximize the patient’s comfort, decrease the length of recovery and minimize pain management requirements, a minimally invasive approach should be used unless contraindicated. If feasible, robotically assisted mitral valve repair, the least invasive form of mitral valve surgery, should be considered for all patients with severe myxomatous MV disease (13-15).
From a surgical perspective, the most important preoperative diagnostic tests are imaging studies including echocardiography, cardiac catheterization, computed tomography (CT) scan and lower extremity Doppler ultrasound. Echocardiography is the most important diagnostic tool for determining mitral valve function and morphology. Transthoracic echocardiography provides information about the mechanism and severity of mitral regurgitation, size and function of the left and right ventricles, size of the left atrium, degree of pulmonary hypertension and presence of other associated valve lesions (16). Doppler evaluation provides quantitative measures of severity of MR (17). Transesophageal echocardiography is even more accurate in providing information about valve morphology, thus enabling a more precise operative plan. It is also an important intraoperative tool for confirming the success of valve repair. Additional diagnostic tests help reveal the presence of comorbidities that would contraindicate certain approaches. Cardiac catheterization is performed in women >45 and men >40 years old to determine coronary anatomy and the presence or absence of coronary artery disease (CAD). If it is present, we favor complete sternotomy so that concomitant CABG may be performed. Patients with no CAD undergo CT scan of thorax and abdomen. If CT scan shows severe aortic or iliofemoral atherosclerosis and calcification, we perform either complete or partial sternotomy. Patients with no evidence of atherosclerosis or calcification on the CT scan are then candidates for robotic approach or anterolateral thoracotomy if there are no other contraindications (18). Femoral Doppler ultrasound helps to determine groin vessel size. Patients with large vessels are suitable for peripheral cannulation, and therefore are candidates for robotic or anterolateral thoracotomy. In patients with small groin vessels (e.g., <0.9 cm) we favor partial sternotomy (Figure 1).
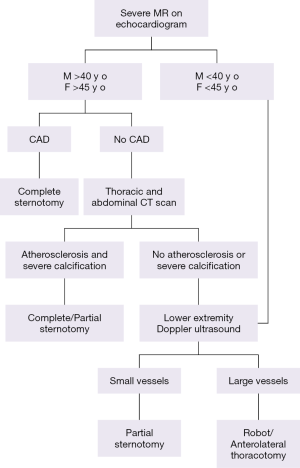
Contraindications for minimally invasive approaches include previous right thoracotomy, chest deformities such as severe pectus excavatum, need for concomitant procedures, presence of severe annular calcification, CAD, significant aortic root or ascending aortic dilatation or calcification, more than mild aortic regurgitation, right ventricular dysfunction, severe pulmonary hypertension, recent (<30 days) myocardial infarction or stroke and others (18). Patients with severe COPD or respiratory insufficiency are also not suitable due to the need for lung isolation with these approaches.
Surgical approaches to mitral valve repair
We approach the valve through complete sternotomy or minimally invasive approaches including partial upper sternotomy, right mini anterolateral thoracotomy and robotically assisted right thoracic approach. Minimally invasive approaches offer comparable quality of MV repair with benefits of less bleeding, decreased need for blood transfusion, shorter ICU and hospital length of stay, less postoperative pain and sternal wound complications, superior cosmetic result, and more rapid resumption of baseline activity (13,14,19). Our practice goal is to perform safe and successful mitral valve surgery through the smallest incision possible.
Complete sternotomy
A vertical, midline incision is made along the whole sternum (incision length is about 16–19 cm). Cardiopulmonary bypass (CPB) is established by cannulating the distal ascending aorta and superior and inferior venae cava (central cannulation). The aorta is cross clamped directly. Myocardial protection is achieved by antegrade cardioplegia (catheter placed directly in the ascending aorta), retrograde cardioplegia (catheter placed in the coronary sinus) and may also include topical or systemic cooling. The mitral valve is exposed through a left atrial incision anterior to the right superior pulmonary vein, going posterior and parallel to interatrial groove (standard left atriotomy).
With increasing application of minimally invasive surgery and the development of simplified repair techniques, this traditional approach is rarely used today in simple MV repair surgery at our institution.
Partial upper sternotomy
In this approach, we use a 6–8 cm midline skin incision and upper partial sternotomy. CPB, aortic cross clamping and myocardial protection are achieved in a similar manner as in complete sternotomy. Mitral valve exposure is obtained through an extended transseptal incision, approached from the right atrium.
Partial upper sternotomy is a good alternative to complete sternotomy in patients with peripheral arterial disease or small femoral vessels, when the robotic approach and right mini thoracotomy are contraindicated.
Right mini anterolateral thoracotomy
A skin incision (4–8 cm) is made along the infra-mammary crease lateral to the nipple and the chest is entered through right fourth intercostal space. CPB is established by peripheral cannulation, and aortic occlusion is accomplished using a transthoracic clamp inserted through the third intercostal space in the midaxillary line. Myocardial protection is achieved with antegrade cardioplegia, and can be supplemented by retrograde cardioplegia (especially if more than mild aortic insufficiency is present) and topical or systemic cooling. The mitral valve is approached via standard left atriotomy and the procedure is performed under direct visualization using long shaft instruments. This approach also includes the use of a double lumen endotracheal tube and lung isolation.
Robotically assisted right thoracic approach
This approach entails five main incisions: a working port incision (4 cm) in the right fourth intercostal space and three additional incisions (1 cm) for right and left robotic arms and the camera port. Additional port for the dynamic left atrial retractor is placed through the fourth or the fifth intercostal space in the mid-clavicular line. Port placement is crucial and may vary depending on patient body habitus. It is particularly important to have sufficient separation between the robotic arms to avoid instrument collisions.
CPB is established by peripheral cannulation and aortic occlusion is achieved with a transthoracic clamp. Alternatively, aortic occlusion can be achieved by using endo aortic balloon inserted percutaneously via peripheral arterial access into ascending aorta. Myocardial protection can include antegrade cardioplegia, percutaneously placed retrograde cardioplegia (especially in cases of more than mild AI), and systemic hypothermia. As with right mini thoracotomy, lung isolation with double lumen endotracheal tube is necessary. We use a robotic surgical system with special robotic instruments.
Pros and cons of surgical approaches
Each of the above described approaches for mitral valve repair has its advantages and limitations. Minimally invasive and conventional approaches are equally safe, with no significant differences in mortality, safety and efficacy, and with outcomes highly dependent on surgeon expertise (10,20,21). Minimally invasive surgical techniques were initially introduced in the mid-1990s with a goal of minimizing surgical trauma while preserving the safety and quality achieved by conventional surgery. Advantages of minimally invasive surgery include enhanced cosmesis, less postoperative pain, shorter ICU and hospital length of stay, better respiratory function, less transfusion requirement, less infectious complications and faster return to work (14). All these reasons have led us to perform over 80% of isolated mitral valve repairs through minimally invasive approaches at our institution. The commonly raised disadvantages of minimally invasive approaches include longer perfusion and cross-clamp times and a greater risk for stroke (9). However, several studies have shown that the risk of stroke is in fact not greater with minimally invasive approaches (20,22,23). In addition, we have demonstrated that myocardial ischemic times, cardiopulmonary bypass times and the rate of complications can be lower or similar with minimally invasive approaches (13).
The least invasive minimally invasive approach is robotically assisted. This approach is as safe and effective as other minimally invasive approaches or complete sternotomy (24). It provides excellent visualization of structures and enhanced spatial orientation compared to other minimally invasive approaches. Possible disadvantages and likely the reasons for the robotic approach not gaining widespread use are the complexity of procedure and the cost associated with greater initial investment, maintenance, disposable instruments and retrograde cardioplegia catheters. It has been suggested that this may be compensated for by the overall economic advantages of robotic approach, specifically shorter hospital stay, faster recovery and faster return to work (18,25).
Mitral valve repair techniques
The 2014 ACC/AHA guidelines suggest that all repairs requiring more than single scallop posterior leaflet repair should be considered complex and managed by more experienced mitral valve surgeons with established outcomes, including acute success rate as well as long-term durability (26).
We have built a decision tree of mitral valve techniques based on our experience that is relatively simple and has led to 98% repair success (Figure 2). This may help improve standardization of techniques for complex mitral valve repair and enable availability of repair in more centers.
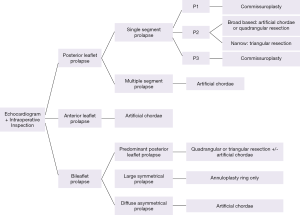
Valve morphology, particularly lesion site and extent, dictates the choice of mitral valve repair technique in degenerative mitral disease. Information obtained by echocardiogram is further confirmed by detailed intraoperative inspection of the specific portions of the mitral valve apparatus. Following segmental anatomical approach to mitral valve pathology, we will discuss repair techniques used at our institution for posterior, anterior, bileaflet and commissural prolapse.
The common feature in all leaflet and chordal repair techniques used at our institution is the use of a partial flexible annuloplasty band.
Posterior leaflet prolapse
Single or multiple segments of the posterior leaflet can be affected. Isolated prolapse or flail of the mid-portion of the posterior leaflet (P2) with annular dilatation is the most common pathology causing mitral regurgitation. Triangular resection is used if the P2 prolapse is narrow-based. Broad-based P2 prolapse is repaired by quadrangular resection or with artificial chordae. Isolated P1 or P3 (commissural prolapse) is simply repaired by commissuroplasty.
Triangular resection
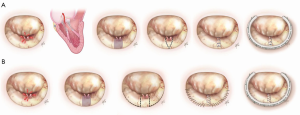
The preferred choice for localized narrow-based P2 repair at our institution is triangular resection (Figure 3A). It involves resection of the prolapsed segment of the leaflet with incisions on the leaflet angled toward one another as the incision approaches the annular level. There is no need for annular plication, and the risk of kinking or distortion of the circumflex artery is decreased. This technique is simple, effective, durable and suitable for small localized prolapse (27).
Quadrangular resection
The quadrangular portion of the posterior leaflet is identified and marked with stay sutures. The diseased portion is then resected and the gap is closed using sliding plasty technique. The leaflet edges are approximated without tension and repair is completed with annuloplasty. This previously widely used technique has fallen out of favor at our institution due to more prevalent use of artificial chordae (Figure 3B).
Chordal replacement
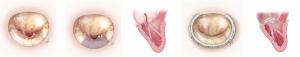
Chordal replacement was introduced experimentally by Frater and colleagues in the early 1980s. Since then it has been widely adopted and used for more complex posterior, anterior and bileaflet repairs. The neochordae are constructed using expanded polytetrafluoroethylene (ePTFE). The neochordal length is established by lowering the height of the prolapsed segment to the level of non prolapsed segments to achieve good leaflet coaptation. We use artificial ePTFE chordae to repair posterior leaflet in cases of broad-based P2 or multi segment prolapse (Figure 4).
Systolic anterior motion
Systolic anterior motion (SAM) is a potential complication that may arise after mitral valve repair. It occurs postoperatively in approximately 5% of patients with myxomatous disease. If not recognized, it can lead to left ventricular outflow obstruction and decreased cardiac output. Patients with preexisting SAM, excessive leaflet tissue, prominent septal buldge and tall and thickened posterior leaflet are considered high risk for developing postoperative SAM. Several techniques have been described to reduce the risk of SAM, including use of sliding plasty, large triangular resection, use of large annuloplasty ring, posterior leaflet ventricularization, and chordal reduction of posterior leaflet (28,29). This list is not complete; however all techniques described so far are directed towards creating a more posteriorly located valve coaptation point and moving the anterior leaflet away from the left ventricular outflow tract.
Anterior leaflet prolapse
Isolated anterior leaflet prolapse comprises approximately one third of degenerative mitral regurgitation cases and is traditionally considered more challenging to repair. This is partially due to less successful long term repair results (30). Prolapse or flail of the middle scallop of the anterior leaflet (A2) is the most common type of anterior leaflet pathology.
Various techniques were developed to deal with anterior leaflet prolapse over the past three decades. Resection techniques, although widely used for repair of the posterior leaflet prolapse, are generally not feasible for anterior repair. Chordal shortening yields less durable results than chordal transfer or replacement (31,32). The chordal transfer technique was favored prior to greater utilization of artificial chordae.
Artificial chordae
Most of the anterior leaflet repair techniques (resection, chordal shortening and chordal transposition) have lost popularity with increasing use of artificial chordae. The simplicity, durability and reproducibility of chordal replacement with artificial ePTFE chordae led to chordae replacement becoming the preferred technique for anterior leaflet repair. Neochordal length is determined by visual estimation of appropriate leaflet coaptation and the overall appearance of the mitral valve apparatus (Figure 5).
Commissural prolapse
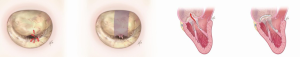
For prolapse of medial (A3/P3) and lateral (A1/P1) scallops of anterior, posterior or both leaflets, we use commissuroplasty (edge-to-edge repair). The free edge of the anterior leaflet is affixed to the free edge of the posterior leaflet, thus reducing the circumference of the mitral orifice. A larger annuloplasty ring is used to avoid mitral stenosis. This is a simple, reproducible and efficient technique (Figure 6).
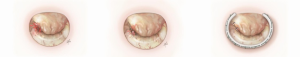
Bileaflet prolapse (Barlow’s disease)
Repair of the bileaflet prolapse has been considered technically challenging. Bileaflet prolapse is a common feature of Barlow’s disease. The term Barlow’s disease refers to myxomatous degeneration that affects the entire valve causing excessive thickening of the leaflet tissue. Chordae are often elongated, thickened, fused and calcified. Annular dilatation with multisegmental prolapse and billowing of the valve are typical findings. These patients have complex valve pathology and dysfunction. Therefore, surgical repair is more complicated and has to address all lesions present in order to achieve good leaflet coaptation and valve competency.
The technique used in cases of bileaflet prolapse with predominant prolapse of the posterior leaflet is triangular resection. If the prolapse is large and symmetrical, we use the annuloplasty ring only to achieve repair. Asymmetrical diffuse prolapse is managed with artificial chordae.
Conclusions
Improved outcomes in the management of degenerative MV disease resulted from evidence based practice changes highlighted in this article, including early surgery before signs of LV function deterioration, preference for MV repair versus replacement and use of minimally invasive technique whenever feasible. High volume centers offer improved outcomes and have higher rates of repair and more experience in minimally invasive approaches. We have presented our institutional approach to preoperative and intraoperative decision making and emphasized the need for more uniform quality across hospitals that would offer a higher chance of better overall outcomes for all patients.
Robotic approach represents the least invasive approach for mitral valve repair that offers multiple advantages including increased operative dexterity, tremor free movements, ambidexterity, lack of fulcrum effect, superior 3D surgical visualization, less transfusion requirement, less infectious complications, better cosmetic result, shorter length of stay and faster return to baseline activities. Main limitations of robotic approach are lack of haptic feedback, longer learning curve, initial cost of equipment and need for specific selection of patients with no contraindications. With advancing technology, we will likely overcome some of these limitations, and thus the robotic approach will have potential to become the universally preferred approach for mitral valve repair in carefully selected patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Freed LA, Levy D, Levine RA, et al. Prevalence and clinical outcome of mitral-valve prolapse. N Engl J Med 1999;341:1-7. [Crossref] [PubMed]
- Enriquez-Sarano M, Schaff HV, Orszulak TA, et al. Valve repair improves the outcome of surgery for mitral regurgitation. A multivariate analysis. Circulation 1995;91:1022-8. [Crossref] [PubMed]
- Beresniak A, Sabatier B, Achouh P, et al. Cost-effectiveness of mitral valve repair versus replacement by biologic or mechanical prosthesis. Ann Thorac Surg 2013;95:98-104. [Crossref] [PubMed]
- Barlow CW, Imber CJ, Sharples LD, et al. Cost implications of mitral valve replacement versus repair in mitral regurgitation. Circulation 1997;96:II-90-3; discussion II-94-5.
- Enriquez-Sarano M, Suri RM, Clavel MA, et al. Is there an outcome penalty linked to guideline-based indications for valvular surgery? Early and long-term analysis of patients with organic mitral regurgitation. J Thorac Cardiovasc Surg 2015;150:50-8. [Crossref] [PubMed]
- Gillinov AM, Mihaljevic T, Blackstone EH, et al. Should patients with severe degenerative mitral regurgitation delay surgery until symptoms develop? Ann Thorac Surg 2010;90:481-8. [Crossref] [PubMed]
- Suri RM, Vanoverschelde JL, Grigioni F, et al. Association between early surgical intervention vs watchful waiting and outcomes for mitral regurgitation due to flail mitral valve leaflets. JAMA 2013;310:609-16. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:e57-185. [Crossref] [PubMed]
- Gammie JS, Sheng S, Griffith BP, et al. Trends in mitral valve surgery in the United States: results from the Society of Thoracic Surgeons Adult Cardiac Surgery Database. Ann Thorac Surg 2009;87:1431-7; discussion 1437-9. [Crossref] [PubMed]
- McClure RS, Athanasopoulos LV, McGurk S, et al. One thousand minimally invasive mitral valve operations: early outcomes, late outcomes, and echocardiographic follow-up. J Thorac Cardiovasc Surg 2013;145:1199-206. [Crossref] [PubMed]
- Kilic A, Shah AS, Conte JV, et al. Operative outcomes in mitral valve surgery: combined effect of surgeon and hospital volume in a population-based analysis. J Thorac Cardiovasc Surg 2013;146:638-46. [Crossref] [PubMed]
- Ad N, Barnett SD, Speir AM, et al. Institutional and national trends in isolated mitral valve surgery over the past decade. Curr Opin Cardiol 2008;23:99-104. [Crossref] [PubMed]
- Mihaljevic T, Jarrett CM, Gillinov AM, et al. Robotic repair of posterior mitral valve prolapse versus conventional approaches: potential realized. J Thorac Cardiovasc Surg 2011;141:72-80.e1-4.
- Svensson LG, Atik FA, Cosgrove DM, et al. Minimally invasive versus conventional mitral valve surgery: a propensity-matched comparison. J Thorac Cardiovasc Surg 2010;139:926-32.e1-2.
- Suri RM, Taggarse A, Burkhart HM, et al. Robotic Mitral Valve Repair for Simple and Complex Degenerative Disease: Midterm Clinical and Echocardiographic Quality Outcomes. Circulation 2015;132:1961-8. [Crossref] [PubMed]
- Zoghbi WA, Enriquez-Sarano M, Foster E, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr 2003;16:777-802. [Crossref] [PubMed]
- Enriquez-Sarano M, Avierinos JF, Messika-Zeitoun D, et al. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. N Engl J Med 2005;352:875-83. [Crossref] [PubMed]
- Suri RM, Dearani JA, Mihaljevic T, et al. Mitral valve repair using robotic technology: Safe, effective, and durable. J Thorac Cardiovasc Surg 2016;151:1450-4. [Crossref] [PubMed]
- Algarni KD, Suri RM, Schaff H. Minimally invasive mitral valve surgery: Does it make a difference? Trends Cardiovasc Med 2015;25:456-65. [Crossref] [PubMed]
- Mihaljevic T, Cohn LH, Unic D, et al. One thousand minimally invasive valve operations: early and late results. Ann Surg 2004;240:529-34; discussion 534. [Crossref] [PubMed]
- Galloway AC, Schwartz CF, Ribakove GH, et al. A decade of minimally invasive mitral repair: long-term outcomes. Ann Thorac Surg 2009;88:1180-4. [Crossref] [PubMed]
- Schneider F, Onnasch JF, Falk V, et al. Cerebral microemboli during minimally invasive and conventional mitral valve operations. Ann Thorac Surg 2000;70:1094-7. [Crossref] [PubMed]
- Holzhey DM, Shi W, Borger MA, et al. Minimally invasive versus sternotomy approach for mitral valve surgery in patients greater than 70 years old: a propensity-matched comparison. Ann Thorac Surg 2011;91:401-5. [Crossref] [PubMed]
- Mihaljevic T, Koprivanac M, Kelava M, et al. Value of robotically assisted surgery for mitral valve disease. JAMA Surg 2014;149:679-86. [Crossref] [PubMed]
- Suri RM, Antiel RM, Burkhart HM, et al. Quality of life after early mitral valve repair using conventional and robotic approaches. Ann Thorac Surg 2012;93:761-9. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Thorac Cardiovasc Surg 2014;148:e1-e132. [Crossref] [PubMed]
- George KM, Mihaljevic T, Gillinov AM. Triangular resection for posterior mitral prolapse: rationale for a simpler repair. J Heart Valve Dis 2009;18:119-21. [PubMed]
- Gillinov AM, Cosgrove DM 3rd. Modified sliding leaflet technique for repair of the mitral valve. Ann Thorac Surg 1999;68:2356-7. [Crossref] [PubMed]
- Suri RM, Burkhart HM, Schaff HV. A novel method of leaflet reconstruction after triangular resection for posterior mitral valve prolapse. Ann Thorac Surg 2010;89:e53-6. [Crossref] [PubMed]
- David TE, Ivanov J, Armstrong S, et al. A comparison of outcomes of mitral valve repair for degenerative disease with posterior, anterior, and bileaflet prolapse. J Thorac Cardiovasc Surg 2005;130:1242-9. [Crossref] [PubMed]
- Phillips MR, Daly RC, Schaff HV, et al. Repair of anterior leaflet mitral valve prolapse: chordal replacement versus chordal shortening. Ann Thorac Surg 2000;69:25-9. [Crossref] [PubMed]
- Smedira NG, Selman R, Cosgrove DM, et al. Repair of anterior leaflet prolapse: chordal transfer is superior to chordal shortening. J Thorac Cardiovasc Surg 1996;112:287-91; discussion 291-2. [Crossref] [PubMed]





