Aortic valve sparing operations: outcomes at 20 years
Aortic valve sparing operations have now been performed for over two decades in our institution (1). There are basically 2 types of aortic valve sparing operations: reimplantation of the aortic valve and remodeling of the aortic root (2,3). This presentation summarizes our clinical experience with the original procedures as we have described them (2,3) ( video 1).
Material and methods
From May 1988 to December 2010, 374 consecutive patients had aortic valve sparing operations at the Peter Munk Cardiac Centre. Table 1 summarizes the patients’ clinical characteristics and Table 2 summarizes the operative data. Patients have been followed prospectively with annual assessment of valve function by echocardiography. For this report the follow-up was closed on December 31, 2011; it was 98.4% complete and the mean duration was 7.2±4.5 years. This study was approved by the Review Ethic Board of University Health Network.
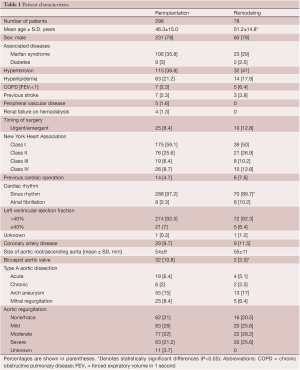
Full table
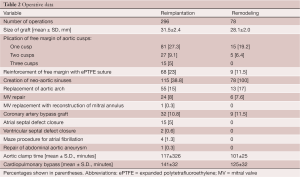
Full table
Results
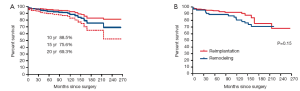
There were 5 deaths within the first 90 days (4 patients had reimplantation and 1 had remodeling). There were 32 late deaths (18 had reimplantation and 14 had remodeling). The overall survival at 10, 15 and 20 years was 88.5%, 75.6% and 69.3% respectively. Figure 1 shows the survival estimates following reimplantation and remodeling procedures. Age by increments of 5 years was the only predictor of mortality from any cause.
Three patients developed infective endocarditis: 1 in the aortic valve (remodeling group) and 2 in the mitral valve (both in the reimplantation group). The patient with aortic valve endocarditis developed an aortic root abscess and was treated with antibiotics and aortic root replacement with an aortic homograft. One patient with mitral valve endocarditis was successfully treated with antibiotics alone and the other also required mitral valve repair because of severe mitral regurgitation. All 3 patients survived.
Including perioperative events 14 patients suffered thromboembolic complications: 4 strokes and 10 TIA’s.
Twenty-nine patients were taking oral anticoagulation at the last follow-up contact because of previous thromboembolic complications or atrial fibrillation. Four patients suffered major hemorrhagic complications, but none was fatal.
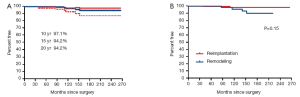
Seven patients have undergone reoperation on the aortic valve, 3 in the reimplantation and 4 in the remodeling group. The aortic valve was re-repaired in one and replaced in 6. The indication for surgery was aortic insufficiency in 6 and endocarditis in 1. The overall freedom from reoperation in the aortic valve at 10, 15 and 20 years were 97.1%, 94.2% and 94.2% respectively. Figure 2 shows the estimates of freedom from reoperation in the aortic valve after reimplantation and remodeling procedures.
Thirteen patients developed moderate aortic insufficiency (AI) and 6 developed severe AI during follow-up. Five of these 19 patients had bicuspid aortic valve disease. The freedom from moderate or severe AI in all patients and after reimplantation and remodeling procedures is shown in Table 3. Remodeling of the aortic root was associated with a higher risk of AI than reimplantation of the aortic valve but the difference did not reach statistical significance by log rank analysis. Age by increments of 5 years, bicuspid aortic valve and hypertension were associated with increased risk of developing moderate or severe AI by univariate analysis but only age by 5 years increments was an independent predictor of AI. Marfan syndrome, moderate or severe AI before surgery, cusp plication and cusp reinforcement with expanded polytetrafluoroethylene suture were not associated with increased risk of postoperative AI by univariate or multivariate analyses.
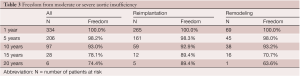
Full table
At the time of the last follow-up contact, 82% of patients were in NYHA functional class I, 13% in class II and 5% in class III.
Comments
The long-term results of aortic valve sparing operations have been excellent in our experience as shown in this study. We continue to use both techniques and try to match the procedure to the pathology of the aortic root. Older patients (e.g., age >50 years) with aortic root aneurysm and normal aortic annulus can be safely treated with the remodeling procedure as long as their aortic annulus is normal. A normal aortic annulus is relatively small (4) and even mild dilatation of the annulus can result in mismatch between areas of the cusps and the aortic valve orifice. Thus, the number of patients suitable for this procedure is relatively small. Most of our patients who had remodeling procedure and developed AI either had Marfan syndrome or incompetent bicuspid aortic valve, two conditions frequently associated with dilatation of the aortic annulus. Even if the aortic annulus is normal in these patients it can dilate later on after the remodeling procedure (5).
Younger patients with inherited aortic root aneurysms such as in Marfan syndrome, Loyes-Dietz syndrome, familial aneurysm, and incompetent bicuspid aortic valve frequently have associated annuloaortic ectasia or develop dilatation of the aortic annulus years after the remodeling of the aortic root and have an increased risk of late AI (6,7). Thus, these patients should have reimplantation of the aortic valve to permanently stabilize the aortic annulus.
Reimplantation of the aortic valve into a cylindrical graft has been used extensively and the long-term results have been excellent as demonstrated in this present study. The diameter of the graft is determined either by estimating the ideal diameter of the sinotubular junction or by measuring the height of the cusps (1,8,9). We often measure the diameter of the aortic annulus, the height of the cusps and estimate the ideal diameter of the sinotubular junction before choosing a graft. De Kerchove and colleagues determine the diameter of the graft by measuring the height of the commissure between the non-coronary cusp and the left coronary cusp (10). Patients with aortic root aneurysm may have cusps larger than normal and dilated aortic annulus, thus no single measurement is adequate to select the diameter of the graft in all cases. If the selected graft is too large it may not reduce the diameter of the aortic annulus to allow for adequate cusp coaptation, and if the graft is too small the cusps may touch the graft during systole with consequent cusp abrasion. In our experience, most patients with aortic root aneurysm need grafts of 30 mm (range of 26 to 34 mm), depending on their body surface areas and the heights of the cusps.
It has been shown that the presence of aortic sinuses is important for normal cusp motion and reduction of cusp stress (11). There is echocardiographic evidence that opening and closure velocity of the cusps are increased when the valve is reimplanted into a cylindrical graft without neo-aortic sinuses and the creation of neo-aortic sinuses reduces this velocity (12). In addition to the aortic sinuses, compliance of aortic root is also important to modulate mechanical stresses on the cusps (13). From this viewpoint, remodeling of the aortic root is physiologically superior to reimplantation of the aortic valve because postoperatively the aortic annulus movements closely resemble the normal (11). However, as stated above remodeling of the aortic root does not correct or prevent annular dilatation and development of late AI is a serious drawback in young patients. Thus, reimplantation remains the procedure of choice to treat patients with inherited aortic root aneurysms. Whether creation of neo-aortic sinuses is important remains to be proven because the longest follow-up on this operation is on patients who had a cylindrical graft and the results have been excellent up to 20 years. Regardless of whether the remodeling of the aortic root or reimplantation of the aortic valve is used, at the end of the procedure the cusps must coapt within the reconstructed aortic root and the cusps must coapt for several millimetres (14,15). In order to accomplish that the free margin of the cusps may have to be shortened by plication along the nodule of Arantius or with reinforcement of the free margin with fine expanded polytetrafluoroethylene sutures.
Bicuspid aortic valve may be associated with aortic root aneurysm and if the cusps are of reasonable quality they can be preserved and provide satisfactory results. Since dilatation of the aortic annulus is often present in patients with incompetent bicuspid aortic valves, the technique of reimplantation of the aortic valve is probably better than other techniques (16).
The main problem after aortic valve sparing operations is the development of AI. It has been established that in young patients with inherited connective tissue disorders reimplantation of the aortic valve provides more stable aortic valve function than remodeling of the aortic root. The main cause of early failure of aortic valve sparing operations is technical errors (17) and probably lack of recognition of cusp prolapse. As mentioned above, regardless of the technique used to repair the dilated aortic root, the cusps must coapt above the level of the nadir of the aortic annulus and the coaptation length must be of at least 4 mm in the central part. The main cause of late failure is probably degeneration of the aortic cusps but more information is needed to confirm this observation. It has been postulated that a rigid aortic root may accelerate degenerative changes in the aortic cusps (13). Interestingly, we have found that age had a protective effect against the development of late AI after reimplantation of the aortic valve, suggesting that elastic aortic cusps probably have greater adaptability to a rigid root than the more sclerotic ones often seen in older patients.
In summary, aortic valve sparing operations to treat patients with aortic root aneurysm with or without aortic insufficiency, and patients with ascending aortic aneurysm and aortic insufficiency, are no longer “experimental” procedures and the principles are well established. Thus, patients with bicuspid or tricuspid aortic valves and an aneurysm can be successfully treated with these procedures. Reimplantation of the aortic valve should be employed in patients with inherited connective tissue disorders associated with annular dilatation. The role of neo-aortic sinuses in the durability of this operation remains to be determined with further clinical follow-up. Remodeling of the aortic root is ideal for older patients with normal aortic annulus and primarily ascending aortic aneurysms. The long term results of these operations have been excellent and justify their inclusion in the surgical armamentarium to treat patients with aortic root and ascending aortic aneurysms.
Acknowledgements
I am indebted to Susan Armstrong for maintaining the follow-up in our patients who had aortic valve sparing procedures and to Cedric Manlhiot for performing the statistical analyses.
Disclosure: The author declares no conflict of interest.
References
- David TE, Feindel CM. An aortic valve-sparing operation for patients with aortic incompetence and aneurysm of the ascending aorta. J Thorac Cardiovasc Surg 1992;103:617-21; discussion 622.
- David TE, Feindel CM, Bos J. Repair of the aortic valve in patients with aortic insufficiency and aortic root aneurysm. J Thorac Cardiovasc Surg 1995;109:345-51; discussion 351-2.
- David TE. Remodeling of the aortic root and preservation of the native aortic valve. Op Tech Cardiac Thorac Surg 1996;1:44-56.
- Capps SB, Elkins RC, Fronk DM. Body surface area as a predictor of aortic and pulmonary valve diameter. J Thorac Cardiovasc Surg 2000;119:975-82.
- de Oliveira NC, David TE, Ivanov J, et al. Results of surgery for aortic root aneurysm in patients with Marfan syndrome. J Thorac Cardiovasc Surg 2003;125:789-96.
- Birks EJ, Webb C, Child A, et al. Early and long-term results of a valve-sparing operation for Marfan syndrome. Circulation 1999;100:II29-35.
- Hanke T, Charitos EI, Stierle U, et al. Factors associated with the development of aortic valve regurgitation over time after two different techniques of valve-sparing aortic root surgery. J Thorac Cardiovasc Surg 2009;137:314-9.
- David TE, Maganti M, Armstrong S. Aortic root aneurysm: principles of repair and long-term follow-up. J Thorac Cardiovasc Surg 2010;140:S14-9; discussion S45-51.
- David TE. How I do aortic valve sparing operations to treat aortic root aneurysm. J Card Surg 2011;26:92-9.
- de Kerchove L, Boodhwani M, Glineur D, et al. A new simple and objective method for graft sizing in valve-sparing root replacement using the reimplantation technique. Ann Thorac Surg 2011;92:749-51.
- Leyh RG, Schmidtke C, Sievers HH, et al. Opening and closing characteristics of the aortic valve after different types of valve-preserving surgery. Circulation 1999;100:2153-60.
- De Paulis R, De Matteis GM, Nardi P, et al. Opening and closing characteristics of the aortic valve after valve-sparing procedures using a new aortic root conduit. Ann Thorac Surg 2001;72:487-94.
- Fokin AA, Robicsek F, Cook JW, et al. Morphological changes of the aortic valve leaflets in non-compliant aortic roots: in-vivo experiments. J Heart Valve Dis 2004;13:444-51.
- Pethig K, Milz A, Hagl C, et al. Aortic valve reimplantation in ascending aortic aneurysm: risk factors for early valve failure. Ann Thorac Surg 2002;73:29-33.
- Kunihara T, Aicher D, Rodionycheva S, et al. Preoperative aortic root geometry and postoperative cusp configuration primarily determine long-term outcome after valve-preserving aortic root repair. J Thorac Cardiovasc Surg 2012;143:1389-95.
- de Kerchove L, Boodhwani M, Glineur D, et al. Valve sparing-root replacement with the reimplantation technique to increase the durability of bicuspid aortic valve repair. J Thorac Cardiovasc Surg 2011;142:1430-8.
- Oka T, Okita Y, Matsumori M, et al. Aortic regurgitation after valve-sparing aortic root replacement: modes of failure. Ann Thorac Surg 2011;92:1639-44.





