Current transcatheter devices to treat functional tricuspid regurgitation with discussion of issues relevant to clinical trial design
Introduction
Functional or secondary tricuspid regurgitation (TR) is believed to be the most common etiology of severe TR in the western world (1,2). The anatomical etiologies of functional or secondary TR have typically included: right ventricular (RV) dilatation, RV or pulmonary hypertension and RV failure (regional or global) (3). The presence of functional TR, either isolated or in combination with left heart disease, is associated with unfavorable natural history (4,5). Current treatment for TR includes the use of optimal medical therapy involving primarily diuretics, or surgery (6). Surgical mortality for isolated tricuspid valve interventions remain higher than for any other single valve surgery (7,8). Combined tricuspid repair at the time of the left-sided disease treatment is recommended in the setting of trans-thoracic echocardiographic tricuspid annular dilatation (>40 mm or >21 mm/m2) (6). Repairing these patients during their initial valve surgery is the only Class I indication for tricuspid intervention, according to the both ACC/AHA and European guidelines (6,9).
However, moderate-to-severe TR is present in 1.6 million US individuals and only a few (<0.5%) within this population currently undergo surgical tricuspid repair or replacement (10). Recognizing the need for early surgical intervention may be difficult for a number of reasons: the underestimation of TR severity under anesthesia (11); the misconception that TR resolves following mitral valve surgery (12,13); the overestimation of surgical risk when concomitant tricuspid valve surgery is performed at the time of mitral valve surgery (14,15); and the under-appreciation of long-term RV function improvement following correction of TR (16,17). Of note, recent studies show that tricuspid valve surgery combined with left heart surgery is not associated with a significant increase in mortality (18), however, re-operative mortality remains high (19).
Transcatheter options for treating patients with significant residual TR following left heart valve interventions has gained importance. In addition, as more left-sided valve disease is treated with transcatheter therapies, the negative impact of TR on survival in these patients (20-22) has under-scored the importance of developing transcatheter solutions. Although a number of procedures have been developed, ongoing issues which may influence clinical trial design (CTD) for these devices remain, including: defining the severity of TR, understanding the pathophysiology of the disease, determining appropriate indications for intervention and describing relevant clinical outcomes for the disease process.
Transcatheter approaches to tricuspid regurgitation
Given advanced echocardiographic imaging techniques allow consistent and accurate real-time imaging of the tricuspid valve (23), transcatheter solutions to TR are now possible with early trials either ongoing or planned.
MitraClip for tricuspid regurgitation
A number of authors have recently reported successful treatment of TR with the MitraClip (Abbott Vascular, Abbott Park, Illinois) (Figure 1A) (24-26). Recently, Vismara et al. (27) developed an ex vivo porcine model of functional TR to determine the optimal placement of the clip. If a single clip is used, grasping the medial segment (near the tips) of the septal and anterior or septal and posterior leaflets resulted in the greatest increase in forward flow. If a 2-clip procedure is anticipated, then grasping the commissure and medial segments of the septal and anterior leaflets allowed for the best post-procedural outcome, ensuring a complete re-establishment of physiological-like hemodynamics. Formal trials using this device are needed.
Caval implants
Patients with severe TR experience symptoms of chronic right heart failure (peripheral edema, ascites, and orthopnea) with congestive hepatopathy, justifying treatment of the upstream effect of severe TR by placing valved stents within the vena cavae. Lauten et al. (28) first reported implantation of two custom-made transcatheter valves into the superior vena cava (SVC) and inferior vena cava (IVC). This resulted in an immediate fall in vena caval pressures, increase in cardiac output, continued improvement in mean caval pressures, improvement in symptoms and normalization of liver function. A similar single-center study, Heterotopic Implantation Of the Edwards-Sapien XT Transcatheter Valve in the Inferior Vena Cava for the Treatment of Severe Tricuspid Regurgitation (HOVER) trial (ClinicalTrials.gov Identifier: NCT02339974) (29), is currently enrolling.
TriCinch System
Numerous studies have shown that in function TR, the tricuspid annulus dilates in the septo-to-lateral direction. Knowing this pathoanatomy, investigators of the TriCinch System (4TECH Cardio, Galway, Ireland) have developed a device that tethers the anteroposterior dimension of the annulus in order to improve coaptation. The delivery system allows trans-femoral fixation of a stainless-steel corkscrew into the anteroposterior tricuspid valve annulus, which is connected through a Dacron band to a self-expanding nitinol stent placed in the hepatic region of the IVC. It has been implanted in a limited number of patients with isolated functional TR, however, no published results are available. The Percutaneous Treatment of tricuspid valve Regurgitation With the TriCinch System™ (PREVENT) trial (ClinicalTrials.gov Identifier: NCT02098200) is currently enrolling in French, Italian, and Swiss centers.
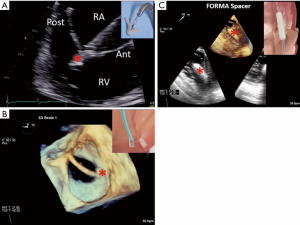
Forma Spacer
A simpler approach to a large regurgitant orifice would be to place a device in the center of the regurgitant orifice, reducing the orifice and forming a surface against which the leaflet tips can coapt. The Forma Spacer (Edwards Lifesciences, Irvine, CA) device is implanted from a left subclavian vein approach, introducing an anchor attached to a foam-filled spacer device (Figure 1B). The anchor is positioned within the RV wall at the apex and the attached spacer is positioned within the central coaptation of the leaflets using echocardiographic guidance. The early report of seven successful implants in high-risk patients with severe TR with no procedural complications (30) has led to a US early feasibility trial (ClinicalTrials.gov Identifier: NCT02471807) which is currently enrolling.
Trialign System
The Trialign System (Mitralign Inc., Tewksbury, Massachusetts) attempts to replicate the results of the modified Kay bicuspidization procedure, which has shown good mid-term (31) and long-term (2) results. Briefly following trans-jugular access, a radiofrequency wire is advanced across the tricuspid annulus from the ventricular side to the right atrium. The ventricular half of a sutured pledget is delivered and cinched (like a venetian blind) in the subannular region, and the other half of the pledget is extruded and cinched on the atrial surface of the tricuspid annulus. These steps are then repeated so that two pledgeted sutures are positioned at the commissures of the posterior leaflet and the two pledgeted sutures plicated, effectively bicuspidizing the tricuspid valve (Figure 1C). Since the first-in-human implantation of their device on the tricuspid annulus (32), a number of devices have been implanted on a compassionate-use basis in Europe. The Early Feasibility of the Mitralign Percutaneous Tricuspid Valve Annuloplasty System (PTVAS) in patients with Chronic Functional Tricuspid Regurgitation (SCOUT) Trial completed enrollment and reported the 30 day results at Transcatheter Cardiovascular Therapeutics (TCT) 2016, showing a significant reduction in tricuspid annular area as well as tricuspid regurgitant orifice with a significant increase in left ventricular stroke volume. Associated with these changes was a significant reduction in New York Heart Association (NYHA) Class, improvement in Minnesota Living with Heart Failure Questionnaire (MLHFQ) and 6-minute walk test (6MWT). Extension of the trial was granted by the FDA and the trial is still enrolling.
Cardioband System
Recent successes and European CE mark approval of the Cardioband System (Valtech Cardio Ltd., Or Yehuda, Israel) for functional mitral regurgitation (FMR) has shown high procedural success with effectiveness in reduction of regurgitation [FMR ≥3+ was reduced from 77.4% to 10.7% 1 month after the procedure (P<0.001) and 13.6% (P<0.001) at 7 months], and was associated with improvement in heart failure symptoms [NYHA functional class III/IV decreased from 95.5% to 18.2% after 7 months (P<0.001); exercise capacity as assessed by 6MWT increased from 250±107 to 332±118 m (P<0.001)] and demonstrated a favorable safety profile (no peri-procedural deaths) (33). The Cardioband implant is a polyester sleeve with radiopaque markers spaced 8 mm apart. The sleeve covers the delivery system that deploys anchors guided by trans-esophageal imaging. A contraction wire is pre-mounted on the Cardioband sleeve and contracting the polyester sleeve from one side is accomplished using a dedicated cinching tool. The tool performs cinching and results in a proportional reduction of the distances between the implanted anchors with significant annular remodeling (34). A number of investigators have successfully performed a modification of the procedure for functional TR in this setting, with initial first-in-man data for its Cardioband Tricuspid (TR) system presented at PCR London Valves 2016. These early compassionate-use cases have shown reductions in annular dimensions, regurgitant volume, orifice areas and mean right atrial pressures. The TRI-REPAIR-CE trial is now enrolling in France and Germany.
Millipede
Another new device still in early development is the Millipede device (Millipede Inc., Stanta Rosa, CA). This device is a complete, adjustable, semi-rigid ring which is attached to the annulus by rotational anchors positioned at defined intervals. The device has a zigzag appearance like the top of a crown, with the anchors at the lowest points and a collar around the hinge-points at the crests. Annular reduction is then accomplished by repositioning the collars further down the crest, effectively reducing the distance between the anchors. Both mitral and tricuspid implants in patients using direct visualization (open surgery) have been performed and were recently presented at PCR London Valves 2016. Early results for the mitral device show both annular reduction, reduction in MR/TR and favorable left ventricular volume reductions, visualized by computed tomography. A trans-septal procedure is currently being developed. In addition to direct reduction in regurgitation with preservation of leaflet architecture, the Millepede device may potentially be used as a docking station for other transcatheter devices such as the balloon-expandable transcatheter valves currently in use for the aortic valve.
Indirect annuloplasty
The trans-atrial intra-pericardial tricuspid annuloplasty (TRAIPTA) device is also currently under investigation, with successful implantation in pre-clinical studies (35). This device is formed from a nitinol wire, pre-shaped into a self-expanding loop to encircle the heart from within the pericardial space. Trans-auricular pericardial access is performed by introducing the device into the right atrium via a trans-femoral vein approach, and using the guidewire to puncture the right atrial appendage. The nitinol loop is then opened and positioned to encircle the heart in the atrio-ventricular groove. The suture is tightened to the desired tension using real-time 1.5-T magnetic resonance imaging (MRI) and the right atrial appendage puncture is closed with an atrial septal occluder. In the initial animal study there were significant reductions in annular area, perimeter and increased tricuspid leaflet-coaptation length. Initial trials in humans may be forthcoming.
Questions about functional tricuspid regurgitation relevant to clinical trial design
The following discussion introduces a number of questions important to future CTD and highlights areas that need further study.
How can we reproducibly determine the severity of tricuspid regurgitation?
Grading of the severity of the TR has been well-described by the American Society of Echocardiography (ASE) (36) and European Association of Echocardiography (EAE) guidelines (37). However, most of the methods used have been poorly-validated resulting in the use of color Doppler visual estimates of severity that have a number of limitations. The use of multiple parameters is frequently required (38). Quantitative assessment of TR with the proximal isovelocity surface area (PISA) method or relative stroke volumes have again not been well studied or validated. The use of three-dimensional (3D) color Doppler, however, shows promise. Velayudhan et al. (39) was one of the first to correlate standard Doppler methods of quantifying severe TR with planimetry of the 3D vena contracta area (VCA): >0.75 cm2 had a sensitivity of 85.2% and specificity of 82.1%. Chen et al. (40) also showed that severe TR by two-dimensional (2D) criteria was associated with a 3D VCA of >0.6±0.4 cm2 and non-severe TR by 2D methods with a 3D VCA of ≤0.3±0.1 cm2. Many of the studies validating the use of these Doppler parameters have significant limitations, with a lack of a “gold standard” for comparison or support from outcomes data. Future studies are needed to determine the reproducibility, accuracy and prognostic utility of echocardiography or cardiac MRI parameters.
What are the predictors of progression/persistence of disease following left heart surgery?
Following isolated mitral valve repair, significant residual TR is observed in up to 40% of patients (41). In patients undergoing concomitant TR repair at the time of mitral surgery, persistent severe TR is still present in 11% at 3 months, and 17% at 5 years (42). Predictors for residual regurgitation after surgical repair have been identified: higher pre-operative TR severity, higher pulmonary artery pressures, mitral replacement rather than repair, worse left ventricular dysfunction and presence of pacemaker leads through the valve area (43). Tricuspid valve morphology may also predict recurrence; tenting height, tenting area (43) and tenting volume (44) are predictors of residual TR following annuloplasty. The current guidelines use a 2D echocardiographic annular measurement to guide intervention (6,9). In keeping with new recommendations for right-heart imaging that call for three different apical views (45), the reproducibility of this measurement will likely be low and the cut-off for severe dilatation may change (46). Advanced imaging techniques to describe the tricuspid valve apparatus, such as multi-slice computed tomography (47) or 3D echocardiography (48), may have greater utility and should be studied. In addition, we currently lack an understanding of the relative contributions of annular dilatation, RV size/function and pulmonary artery pressures on severity of disease, as well as recurrence following intervention and outcomes. Lee et al. (5) looked at the outcome in 813 patients with severe, unrepaired TR and found the 5-year survival was 74% and the predictors of mortality included absolute TR jet area (a surrogate for TR severity), RV function and pulmonary hypertension. How these factors affect outcomes and recurrence of disease will help determine the appropriate patient population for clinical trials of transcatheter devices.
Can early intervention in the disease process (mild or moderate tricuspid regurgitation) affect outcomes?
In light of the recent evidence showing that untreated TR might be associated with worse prognosis (4,49) and that progression of TR following isolated left heart valve surgery is highly likely (50,51) some investigators have suggested that prophylactic treatment of < severe TR is warranted. In fact, guideline recommendations for surgery are currently based upon expert opinion in the absence of evidence-based trial data and support intervening on the tricuspid valve for a dilated tricuspid annulus of ≥40 mm or >21 mm/m2, independent of TR severity (6,9). The target population for prophylactic repair may be patients with mild or moderate TR in the setting of annular dilatation, although studies to test this hypothesis are ongoing (52).
What outcomes are the most relevant for this disease process?
Because of the long natural history of the disease, studies on outcomes may need to focus on soft endpoints. This could include re-hospitalizations, functional status, quality of life or other secondary anatomic/hemodynamic parameters that determine outcomes (i.e., RV function and pulmonary artery pressures). Besides an assessment of functional NYHA Class, disease-specific questionnaires such as the MLHFQ (53) may play a significant role in future trial designs. Tests for validity, reliability and responsiveness were very positive for MLHFQ in a recent study of patients undergoing heart valve surgery (54). A MLHFQ score of less than 24 signifies good health, 24–45 moderate health and 45–105 poor health (53,55). In addition to patient-reported symptoms, tests such as the 6MWT, per the American Thoracic Society Guidelines [2002] (56), have been used to assess quality of life.
Conclusions
Functional TR is associated with increased mortality if untreated, however, current surgical solutions do have their limitations. Transcatheter solutions may be justified, although the disease itself has not been extensively studied and the interaction between annular dilatation, right heart disease and pulmonary hypertension is poorly understood. A number of questions remain in relation to our poor understanding, which may affect the design of clinical trials for this disease.
Acknowledgements
None.
Footnote
Conflicts of Interest: RT Hahn has received speaker honoraria from Edwards Lifesciences, St. Jude Medical and Boston Scientific; and research grant from Philips Healthcare.
References
- Cohen SR, Sell JE, McIntosh CL, et al. Tricuspid regurgitation in patients with acquired, chronic, pure mitral regurgitation. II. Nonoperative management, tricuspid valve annuloplasty, and tricuspid valve replacement. J Thorac Cardiovasc Surg 1987;94:488-97. [PubMed]
- Taramasso M, Vanermen H, Maisano F, et al. The growing clinical importance of secondary tricuspid regurgitation. J Am Coll Cardiol 2012;59:703-10. [Crossref] [PubMed]
- Shah PM, Raney AA. Tricuspid valve disease. Curr Probl Cardiol 2008;33:47-84. [Crossref] [PubMed]
- Nath J, Foster E, Heidenreich PA. Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol 2004;43:405-9. [Crossref] [PubMed]
- Lee JW, Song JM, Park JP, et al. Long-term prognosis of isolated significant tricuspid regurgitation. Circ J 2010;74:375-80. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:e57-185. [Crossref] [PubMed]
- Kilic A, Saha-Chaudhuri P, Rankin JS, et al. Trends and outcomes of tricuspid valve surgery in North America: an analysis of more than 50,000 patients from the Society of Thoracic Surgeons database. Ann Thorac Surg 2013;96:1546-52; discussion 1552. [Crossref] [PubMed]
- Beckmann A, Funkat AK, Lewandowski J, et al. Cardiac surgery in Germany during 2012: a report on behalf of the German Society for Thoracic and Cardiovascular Surgery. Thorac Cardiovasc Surg 2014;62:5-17. [Crossref] [PubMed]
- Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012): the Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur J Cardiothorac Surg 2012;42:S1-44. [Crossref] [PubMed]
- Stuge O, Liddicoat J. Emerging opportunities for cardiac surgeons within structural heart disease. J Thorac Cardiovasc Surg 2006;132:1258-61. [Crossref] [PubMed]
- Frater R. Tricuspid insufficiency. J Thorac Cardiovasc Surg 2001;122:427-9. [Crossref] [PubMed]
- Porter A, Shapira Y, Wurzel M, et al. Tricuspid regurgitation late after mitral valve replacement: clinical and echocardiographic evaluation. J Heart Valve Dis 1999;8:57-62. [PubMed]
- Onorati F, Santarpino G, Marturano D, et al. Successful surgical treatment of chronic ischemic mitral regurgitation achieves left ventricular reverse remodeling but does not affect right ventricular function. J Thorac Cardiovasc Surg 2009;138:341-51. [Crossref] [PubMed]
- Di Mauro M, Bivona A, Iacò AL, et al. Mitral valve surgery for functional mitral regurgitation: prognostic role of tricuspid regurgitation. Eur J Cardiothorac Surg 2009;35:635-9; discussion 639-40. [Crossref] [PubMed]
- Pfannmueller B, Verevkin A, Borger MA, et al. Role of tricuspid valve repair for moderate tricuspid regurgitation during minimally invasive mitral valve surgery. Thorac Cardiovasc Surg 2013;61:386-91. [Crossref] [PubMed]
- Desai RR, Vargas Abello LM, Klein AL, et al. Tricuspid regurgitation and right ventricular function after mitral valve surgery with or without concomitant tricuspid valve procedure. J Thorac Cardiovasc Surg 2013;146:1126-1132.e10. [Crossref] [PubMed]
- Chikwe J, Itagaki S, Anyanwu A, et al. Impact of Concomitant Tricuspid Annuloplasty on Tricuspid Regurgitation, Right Ventricular Function, and Pulmonary Artery Hypertension After Repair of Mitral Valve Prolapse. J Am Coll Cardiol 2015;65:1931-8. [Crossref] [PubMed]
- Badhwar V, Rankin JS, He M, et al. Performing Concomitant Tricuspid Valve Repair at the Time of Mitral Valve Operations Is Not Associated With Increased Operative Mortality. Ann Thorac Surg 2017;103:587-593. [Crossref] [PubMed]
- Bernal JM, Morales D, Revuelta C, et al. Reoperations after tricuspid valve repair. J Thorac Cardiovasc Surg 2005;130:498-503. [Crossref] [PubMed]
- Lindman BR, Maniar HS, Jaber WA, et al. Effect of tricuspid regurgitation and the right heart on survival after transcatheter aortic valve replacement: insights from the Placement of Aortic Transcatheter Valves II inoperable cohort. Circ Cardiovasc Interv 2015.8. [PubMed]
- Ohno Y, Attizzani GF, Capodanno D, et al. Association of tricuspid regurgitation with clinical and echocardiographic outcomes after percutaneous mitral valve repair with the MitraClip System: 30-day and 12-month follow-up from the GRASP Registry. Eur Heart J Cardiovasc Imaging 2014;15:1246-55. [Crossref] [PubMed]
- Frangieh AH, Gruner C, Mikulicic F, et al. Impact of percutaneous mitral valve repair using the MitraClip system on tricuspid regurgitation. EuroIntervention 2016;11:e1680-6. [Crossref] [PubMed]
- Hahn RT. State-of-the-Art Review of Echocardiographic Imaging in the Evaluation and Treatment of Functional Tricuspid Regurgitation. Circ Cardiovasc Imaging 2016.9. [PubMed]
- Hammerstingl C, Schueler R, Malasa M, et al. Transcatheter treatment of severe tricuspid regurgitation with the MitraClip system. Eur Heart J 2016;37:849-53. [Crossref] [PubMed]
- Wengenmayer T, Zehender M, Bothe W, et al. First transfemoral percutaneous edge-to-edge repair of the tricuspid valve using the MitraClip system. EuroIntervention 2016;11:1541-4. [Crossref] [PubMed]
- Braun D, Nabauer M, Massberg S, et al. Transcatheter Repair of Primary Tricuspid Valve Regurgitation Using the MitraClip System. JACC Cardiovasc Interv 2016;9:e153-4. [Crossref] [PubMed]
- Vismara R, Gelpi G, Prabhu S, et al. Transcatheter Edge-to-Edge Treatment of Functional Tricuspid Regurgitation in an Ex Vivo Pulsatile Heart Model. J Am Coll Cardiol 2016;68:1024-33. [Crossref] [PubMed]
- Lauten A, Ferrari M, Hekmat K, et al. Heterotopic transcatheter tricuspid valve implantation: first-in-man application of a novel approach to tricuspid regurgitation. Eur Heart J 2011;32:1207-13. [Crossref] [PubMed]
- O'Neill BP, Wheatley G, Bashir R, et al. Study design and rationale of the heterotopic implantation of the Edwards-Sapien XT transcatheter valve in the inferior VEna cava for the treatment of severe tricuspid regurgitation (HOVER) trial. Catheter Cardiovasc Interv 2016;88:287-93. [Crossref] [PubMed]
- Campelo-Parada F, Perlman G, Philippon F, et al. First-in-Man Experience of a Novel Transcatheter Repair System for Treating Severe Tricuspid Regurgitation. J Am Coll Cardiol 2015;66:2475-83. [Crossref] [PubMed]
- Ghanta RK, Chen R, Narayanasamy N, et al. Suture bicuspidization of the tricuspid valve versus ring annuloplasty for repair of functional tricuspid regurgitation: midterm results of 237 consecutive patients. J Thorac Cardiovasc Surg 2007;133:117-26. [Crossref] [PubMed]
- Schofer J, Bijuklic K, Tiburtius C, et al. First-in-human transcatheter tricuspid valve repair in a patient with severely regurgitant tricuspid valve. J Am Coll Cardiol 2015;65:1190-5. [Crossref] [PubMed]
- Nickenig G, Hammerstingl C, Schueler R, et al. Transcatheter Mitral Annuloplasty in Chronic Functional Mitral Regurgitation: 6-Month Results With the Cardioband Percutaneous Mitral Repair System. JACC Cardiovasc Interv 2016;9:2039-2047. [Crossref] [PubMed]
- Arsalan M, Agricola E, Alfieri O, et al. Effect of Transcatheter Mitral Annuloplasty With the Cardioband Device on 3-Dimensional Geometry of the Mitral Annulus. Am J Cardiol 2016;118:744-9. [Crossref] [PubMed]
- Rogers T, Ratnayaka K, Sonmez M, et al. Transatrial intrapericardial tricuspid annuloplasty. JACC Cardiovasc Interv 2015;8:483-91. [Crossref] [PubMed]
- Zoghbi WA, Enriquez-Sarano M, Foster E, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr 2003;16:777-802. [Crossref] [PubMed]
- Lancellotti P, Moura L, Pierard LA, et al. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: mitral and tricuspid regurgitation (native valve disease). Eur J Echocardiogr 2010;11:307-32. [Crossref] [PubMed]
- Grant AD, Thavendiranathan P, Rodriguez LL, et al. Development of a consensus algorithm to improve interobserver agreement and accuracy in the determination of tricuspid regurgitation severity. J Am Soc Echocardiogr 2014;27:277-84. [Crossref] [PubMed]
- Velayudhan DE, Brown TM, Nanda NC, et al. Quantification of tricuspid regurgitation by live three-dimensional transthoracic echocardiographic measurements of vena contracta area. Echocardiography 2006;23:793-800. [Crossref] [PubMed]
- Chen TE, Kwon SH, Enriquez-Sarano M, et al. Three-dimensional color Doppler echocardiographic quantification of tricuspid regurgitation orifice area: comparison with conventional two-dimensional measures. J Am Soc Echocardiogr 2013;26:1143-52. [Crossref] [PubMed]
- Matsunaga A, Duran CM. Progression of tricuspid regurgitation after repaired functional ischemic mitral regurgitation. Circulation 2005;112:I453-7. [PubMed]
- Navia JL, Nowicki ER, Blackstone EH, et al. Surgical management of secondary tricuspid valve regurgitation: annulus, commissure, or leaflet procedure? J Thorac Cardiovasc Surg 2010;139:1473-1482.e5. [Crossref] [PubMed]
- Fukuda S, Song JM, Gillinov AM, et al. Tricuspid valve tethering predicts residual tricuspid regurgitation after tricuspid annuloplasty. Circulation 2005;111:975-9. [Crossref] [PubMed]
- Min SY, Song JM, Kim JH, et al. Geometric changes after tricuspid annuloplasty and predictors of residual tricuspid regurgitation: a real-time three-dimensional echocardiography study. Eur Heart J 2010;31:2871-80. [Crossref] [PubMed]
- Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1-39.e14. [Crossref] [PubMed]
- Dreyfus J, Durand-Viel G, Raffoul R, et al. Comparison of 2-Dimensional, 3-Dimensional, and Surgical Measurements of the Tricuspid Annulus Size: Clinical Implications. Circ Cardiovasc Imaging 2015;8:e003241. [Crossref] [PubMed]
- Kabasawa M, Kohno H, Ishizaka T, et al. Assessment of functional tricuspid regurgitation using 320-detector-row multislice computed tomography: risk factor analysis for recurrent regurgitation after tricuspid annuloplasty. J Thorac Cardiovasc Surg 2014;147:312-20. [Crossref] [PubMed]
- Spinner EM, Buice D, Yap CH, et al. The effects of a three-dimensional, saddle-shaped annulus on anterior and posterior leaflet stretch and regurgitation of the tricuspid valve. Ann Biomed Eng 2012;40:996-1005. [Crossref] [PubMed]
- Taramasso M, Maisano F, De Bonis M, et al. Prognostic Impact and Late Evolution of Untreated Moderate (2/4+) Functional Tricuspid Regurgitation in Patients Undergoing Aortic Valve Replacement. J Card Surg 2016;31:9-14. [Crossref] [PubMed]
- Dreyfus GD, Corbi PJ, Chan KM, et al. Secondary tricuspid regurgitation or dilatation: which should be the criteria for surgical repair? Ann Thorac Surg 2005;79:127-32. [Crossref] [PubMed]
- Van de Veire NR, Braun J, Delgado V, et al. Tricuspid annuloplasty prevents right ventricular dilatation and progression of tricuspid regurgitation in patients with tricuspid annular dilatation undergoing mitral valve repair. J Thorac Cardiovasc Surg 2011;141:1431-9. [Crossref] [PubMed]
- Pozzoli A, Elisabetta L, Vicentini L, et al. Surgical indication for functional tricuspid regurgitation at initial operation: judging from long term outcomes. Gen Thorac Cardiovasc Surg 2016;64:509-16. [Crossref] [PubMed]
- Supino PG, Borer JS, Franciosa JA, et al. Acceptability and psychometric properties of the Minnesota Living With Heart Failure Questionnaire among patients undergoing heart valve surgery: validation and comparison with SF-36. J Card Fail 2009;15:267-77. [Crossref] [PubMed]
- Holmes C, Briffa N. Patient-Reported Outcome Measures (PROMS) in patients undergoing heart valve surgery: why should we measure them and which instruments should we use? Open Heart 2016;3:e000315. [Crossref] [PubMed]
- Garin O, Herdman M, Vilagut G, et al. Assessing health-related quality of life in patients with heart failure: a systematic, standardized comparison of available measures. Heart Fail Rev 2014;19:359-67. [Crossref] [PubMed]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111-7. [Crossref] [PubMed]





