Complications after aortic valve repair and valve-sparing procedures
Introduction
In patients with chronic isolated aortic insufficiency (AI), surgical indications are based on presence of symptoms, severity of AI, left ventricle (LV) dysfunction or severe LV dilatation. Once deemed necessary, surgery will usually consist of valve replacement with a mechanical or a biological prosthesis. However, aortic valve (AV) repair can now be considered in surgical centers that have developed the appropriate technical expertise, gained experience in patient selection and have demonstrated outcomes equivalent to those obtained with AV replacement (1,2).
Repairing the AV is associated with low mortality, acceptable durability, and a low risk of valve-related events such as endocarditis, hemorrhage and thromboembolism (3,4).
In this paper, we review our 15 years’ experience in 475 patients, that has grown as a learning curve, and we detail some complications that have driven us to refine the surgical techniques. These complications can be divided into immediate unsatisfactory repair necessitating a second cardiopulmonary bypass (CPB) run (n=26, 5.5%), short-term reoperations (during hospital stay) (n=7, 1.5%), and long-term reoperations (n=21, 4.4%) (4).
Echocardiographic evaluation during AV repair
As for the mitral valve (MV), intraoperative transesophageal echocardiography (TEE) is of paramount value to appreciate the feasibility of the repair. Besides considerations related to the patient (age, fibro-elastic diseases, etc), TEE will allow analysis of the mechanism of the AI, evaluating the number and mobility of the cusps, the quality of the cuspal tissue, and also permit aortic root measurements (5). The recent development of a functional classification of the causes of AI has greatly helped in progressive standardization of techniques for surgical repair. Such a classification is based on the mechanism of the regurgitation and links the various causes of AI to a specific surgical technique (6). A good correlation has been found intraoperatively between echocardiographic and surgical evaluation of the aortic valve (7).
Basically, AI can be provoked by (i) a dilatation of the functional aortic annulus (FAA), that constitutes the borders of the aortic root (Type I) with an ensuing tethering of the aortic cups and a central regurgitant jet, (ii) a prolapse of one or 2 cusps (Type II) with an eccentric regurgitant jet directed towards the opposite direction (8), or (iii) cusp restriction where cuspal stiffness or fibrosis precludes adequate cusp coaptation (Type III).
Aortic valve repair should aim at preserving normal cusp mobility while restoring normal geometry to both the FAA and the cusps and offering a sufficient surface of coaptation. Post cardiopulmonary bypass intraoperative echocardiography evaluates the quality of the repair and has a predictive value on its durability (9).
Immediate unsatisfactory repair
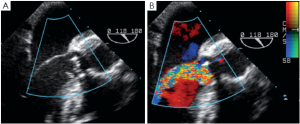
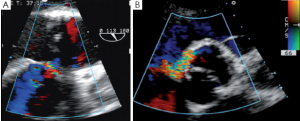
Immediately after aortic unclamping, TEE will disclose any residual defect of coaptation. The pressure applied by the CPB flow through the arterial cannula into the aortic root will cause regurgitation into the left ventricle if the repaired cusps do not coapt adequately (10). The severity of the regurgitation at that moment is close to the one that will be observed after weaning of CPB (Figure 1).
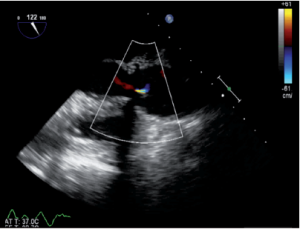
Low coaptation
It has been demonstrated that the 3 most important parameters for a durable repair are: the absence of any or more than mild residual AI, a coaptation length greater than 4 mm and coaptation starting well above the plane of the aortic annulus (9). The coaptation height is the most important parameter (9,11,12) and can be measured intraoperatively with a special caliper. This effective height (eH) has been shown to correlate with the coaptation length measured with TEE in the mid-esophageal long axis view (13,14). Resuspension of the aortic commissures too low into the tube graft leads to early valve failure (11). Therefore, low coaptation observed after a valve-sparing procedure should prompt the surgeon to go back on pump and further plicate the free margin of the billowing cusp(s) in order to increase the height (15) (Figure 2).
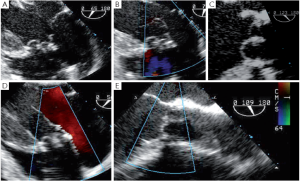
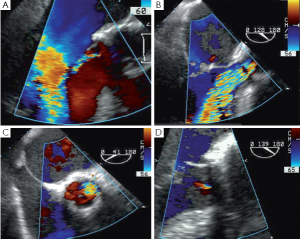
Residual prolapse
Any residual eccentric jet after valve repair and/or valve-sparing surgery evokes the likelihood of a residual cusp prolapse and should also be corrected immediately. Such a prolapse can have two causes: either the prolapse was preexisting and has been undetected or under-corrected, or a prolapse has been surgically induced during the reimplantation procedure. This occurs in the severely chronically dilated aortic root with stretched aortic cusps or in Type 1 bicuspid aortic valves (BAV) (16) with a prominent conjoint cusp (Figure 3). The reduction of either the ventriculo-aortic junction (VAJ) or the sino-tubular junction (STJ) by the tube graft will change the relationship between the free margin length of the cusp and the length of its insertion, hence creating a prolapse. Another induced cause of prolapse can be related to commissural repositioning during valve-sparing surgery that does not respect (intentionally or not) the position of the commissures as they were in the native aortic root.
High gradient
Aortic valve repair implies stabilization of the FAA and hence can provoke some degree of restriction in valve opening. Also, even after repair, a bicuspid valve will still have a dome opening that somehow limits passage of flow. For these reasons, a mild gradient is often observed after valve repair. The gradient measured in the operating room is generally confirmed postoperatively. A gradient of the same magnitude as that observed after valve replacement is acceptable with peak values less than 15-20 mmHg. Because hemodynamics are highly variable during anesthesia, the gradient should be measured when the patient is stabilized and has recovered to normal blood pressure.
Cusp perforation, missed fenestration
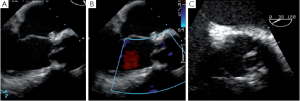
Removal of a fibrotic raphé or a piece of calcium from a cusp can leave a perforation that, if uncorrected, will appear as a residual jet originating both in the short and the long axis from the body of one of the aortic cusps (Figure 4). Depending on the size of the perforation, either a direct suture or a pericardial patch can be used for closure.
Cusps that have been elongated by a chronic dilatation of the aortic root are often thinner and prone to having fenestrations. Small commissural fenestrations do not always need surgical correction, as they may not induce AI. Large fenestrations parallel to the free margin of the cusp can be closed with a PTFE over and over suture that reinforces and resuspends the free margin (17). In rare cases, large fenestrations extend deep towards the body of the cusp; these are closed with a pericardial patch.
Patch dehiscence
Pericardial patches are sometimes used to increase the length of the aortic cusps (18,19) or to close the triangular defect left in a bicuspid valve after removal of a fibrotic raphé. These patches have a poor long-term evolution because they become stiffer with time (20,21). In one of our patients with incompetent BAV and dilatation of the aortic root, we performed a reimplantation procedure and repaired the valve with a tricuspid autologous patch. Unfortunately, dehiscence of the patch occurred in the early postoperative period, necessitating an early reintervention 10 days after the initial repair (Figure 5). In this case, the patch was reattached. The repair lasted for 8 years, after which calcification of the valve necessitated its replacement.
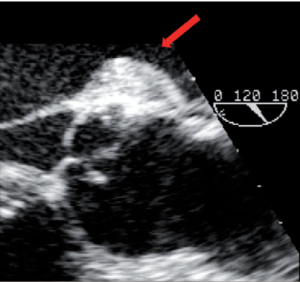
Aortic cusp retraction
Valve-sparing procedures, either remodeling or reimplantation, imply coronary artery reimplantation into the tube graft. Bleeding sometimes occurs at the level of this anastomosis. In a patient presenting continuous bleeding precluding sternal closure after a reimplantation procedure, an additional stitch was placed at the level of the left main coronary artery suture on the tube graft, correcting the problem. By chance, a rapid TEE overview before probe removal disclosed a severe AI due to retraction of the left coronary cusp by the recently added hemostatic stitch (Figure 6). The patient was placed back on CPB, the causal stitch was removed, rendering a normal motion to the left coronary cusp.
Perforation of the base of the anterior mitral leaflet
In two patients, a mild jet was observed through a small perforation at the base of the anterior leaflet of the mitral valve after a reimplantation procedure. That perforation was not present preoperatively and the aorta was immediately re-clamped and a suture stitch was added on the base of the tube graft, closing the perforation, without causing any distortion of the mitral leaflet.
Hematoma
The proximal suture of either a prosthetic valve, a subcommissural annuloplasty (SCA) or a tube graft at the level of the VAJ sometimes causes a posterior hematoma that usually resorbs after a few days (Figure 7).
Complications related to subcommissural annuloplasties
Subcommissural annuloplasties (SCAs) are felted sutures applied at the mid-level of the intercommissural triangles, with the aim of reducing and stabilizing the VAJ. These sutures can also help to increase cusp coaptation (Figure 8).
Disruption
This patient exhibited residual AI after repair of his tricuspid aortic valve and FAA stabilization by 2 SCAs and plication of the STJ. A second run of CPB was initiated and at aortic reopening the SCA placed between the non-coronary cusp (NCC) and the left coronary cusp (LCC) was noted as being untied. A new felted suture was then added in the subcommissural triangle.
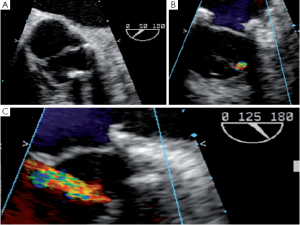
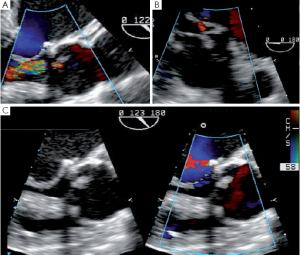
Fistula
SCAs can also provoke a tear or a fistula if the aortic wall tissue is abnormally fragile. A patient with bicuspid aortic valve presented with an aorta to right ventricle fistula at the anterior commissure 6 days after repair. At the immediate post-CPB intraoperative TEE the fistula was not observed, but at discharge transthoracic echocardiography disclosed a severe left to right shunt (Figure 9). The patient was immediately reoperated. The fistula was directly closed and the SCA redone.
Pseudoaneurysm
A more severe complication of SCAs is the acute occurrence of a pseudoaneurysm. Such a pseudoaneurysm can rapidly increase due to a continuous flow from the ascending aorta into the posterior aortic wall (Figure 10). We observed this complication in a case of tricuspid aortic valve repair. The surgery initially consisted of free margin plication associated with three SCAs. After unclamping, a rapidly-growing pseudoaneurysm was detected in the posterior aortic wall (non coronary sinus). At reopening of the aorta we found that the felt of the NCC/LCC SCA got through the aortic wall. Therefore we decided to perform reimplantation of the valve to exclude the abnormally fragile tissues of the aortic root.
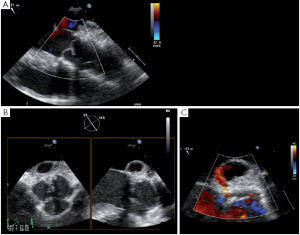
Late complications after AV sparing surgery
Aortic insufficiency due to persistent annular dilatation
Although valve repair is considered an acceptable alternative to replacement for AV incompetence, recurrent AI remains the main concern that limits application of this technique. In our experience, we have demonstrated that valve-sparing root replacement with the reimplantation technique can significantly increase durability of the BAV repair compared to valve repair associated with SCAs (22). We found at the echocardiographic review of repair failures that the VAJ can continue to slowly dilate over the years after SCA, which explains some instances of recurrent regurgitation. We also hypothesize that the better durability observed in the reimplantation technique may not be only due to the VAJ annuloplasty induced by this technique. Indeed, we observed lower transvalvular gradients after the reimplantation technique in comparison to SCA. This observation results from improved cusp mobility due to the reshaping of the FAA and the valve during reimplantation. Similarly, Giebels and colleagues (23) have also reported better durability of valve repair associated with the remodeling technique, suggesting that both valve-sparing procedures induce beneficial effects on the repair that could be: a better stabilization of the FAA, and an improved valve configuration.
This superiority of valve sparing surgery over SCAs associated with cusp repair in BAV has not been demonstrated in tricuspid valves. However, in the presence of a large VAJ (>29 mm), good stabilization should be achieved with either a reimplantation technique or with a prosthesis-based external or internal annuloplasty (24,25).
Aortic insufficiency due to increasing residual prolapse
Recurrent AI can also be due to a residual prolapse that continues to aggravate years after surgery. A coaptation that is too short or too low into the tube graft will lead to a recurrent AI necessitating reoperation. Sometimes the valve is still amenable to further repair; sometimes it will have to be replaced. In our experience re-repair has been performed in 7 patients (25% of the reoperations).
Endocarditis
In a small number of patients, endocarditis can occur after valve repair. In our experience, this appeared in 4 patients (0.19% per year).
Bleeding and thromboembolic (TE) events
Bleeding events have an occurrence rate of 0.23% per year, while TE events account for 0.7% per year.
Progression of the fibrosis and calcification of the valve
Sometimes, and mainly after attempts to repair Type III causes of AI, the causative disease continues to progress, leading to an increased gradient through the repaired valve.
Conclusions
Aortic valve repair can now be performed with very good intermediate and long-term results. The surgical technique needs to be perfect and is becoming more and more standardized. Complications can occur immediately in the operating room, emphasizing the need for a continuous echocardiographic evaluation up to sternal closure. With the patient still on the operating table, it remains possible to go back onto CPB to immediately correct the problem.
Long-term complications are sometimes due to suboptimal technique and do not necessarily need to be addressed by a valve replacement. It is sometimes possible to further repair the valve, offering the patient some more years with low risk of valve-related events.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- American College of Cardiology/American Heart Association Task Force on Practice Guidelines, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation 2006;114:e84-231.
- Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC), European Association for Cardio-Thoracic Surgery (EACTS), Vahanian A, et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 2012;33:2451-96.
- David TE. The aortic valve-sparing operation. J Thorac Cardiovasc Surg 2011;141:613-5.
- Price J, De Kerchove L, Glineur D, et al. Risk of valve-related events after aortic valve repair. Ann Thorac Surg 2012. [Epub ahead of print].
- Van Dyck MJ, Watremez C, Boodhwani M, et al. Transesophageal echocardiographic evaluation during aortic valve repair surgery. Anesth Analg 2010;111:59-70.
- Boodhwani M, de Kerchove L, Glineur D, et al. Repair-oriented classification of aortic insufficiency: impact on surgical techniques and clinical outcomes. J Thorac Cardiovasc Surg 2009;137:286-94.
- le Polain de Waroux JB, Pouleur AC, Goffinet C, et al. Functional anatomy of aortic regurgitation: accuracy, prediction of surgical repairability, and outcome implications of transesophageal echocardiography. Circulation 2007;116:I264-9.
- Boodhwani M, de Kerchove L, Watremez C, et al. Assessment and repair of aortic valve cusp prolapse: implications for valve-sparing procedures. J Thorac Cardiovasc Surg 2011;141:917-25.
- le Polain de Waroux JB, Pouleur AC, Robert A, et al. Mechanisms of recurrent aortic regurgitation after aortic valve repair: predictive value of intraoperative transesophageal echocardiography. JACC Cardiovasc Imaging 2009;2:931-9.
- Koshy T, Misra S, Sinha PK, et al. A novel technique to assess aortic valve repair before releasing the aortic cross-clamp. J Cardiothorac Vasc Anesth 2009;23:79-81.
- Pethig K, Milz A, Hagl C, et al. Aortic valve reimplantation in ascending aortic aneurysm: risk factors for early valve failure. Ann Thorac Surg 2002;73:29-33.
- Aicher D, Langer F, Lausberg H, et al. Aortic root remodeling: ten-year experience with 274 patients. J Thorac Cardiovasc Surg 2007;134:909-15.
- Schäfers HJ, Aicher D, Langer F, et al. Preservation of the bicuspid aortic valve. Ann Thorac Surg 2007;83:S740-5; discussion S785-90.
- Bierbach BO, Aicher D, Issa OA, et al. Aortic root and cusp configuration determine aortic valve function. Eur J Cardiothorac Surg 2010;38:400-6.
- Boodhwani M, de Kerchove L, Glineur D, et al. A simple method for the quantification and correction of aortic cusp prolapse by means of free margin plication. J Thorac Cardiovasc Surg 2010;139:1075-7.
- Sievers HH, Schmidtke C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg 2007;133:1226-33.
- de Kerchove L, Boodhwani M, Glineur D, et al. Cusp prolapse repair in trileaflet aortic valves: free margin plication and free margin resuspension techniques. Ann Thorac Surg 2009;88:455-61; discussion 461.
- Duran CM, Gometza B, Shahid M, et al. Treated bovine and autologous pericardium for aortic valve reconstruction. Ann Thorac Surg 1998;66:S166-9.
- Lausberg HF, Aicher D, Langer F, et al. Aortic valve repair with autologous pericardial patch. Eur J Cardiothorac Surg 2006;30:244-9.
- Boodhwani M, de Kerchove L, Glineur D, et al. Repair of regurgitant bicuspid aortic valves: a systematic approach. J Thorac Cardiovasc Surg 2010;140:276-284.e1.
- Aicher D, Kunihara T, Abou Issa O, et al. Valve configuration determines long-term results after repair of the bicuspid aortic valve. Circulation 2011;123:178-85.
- de Kerchove L, Boodhwani M, Glineur D, et al. Valve sparing-root replacement with the reimplantation technique to increase the durability of bicuspid aortic valve repair. J Thorac Cardiovasc Surg 2011;142:1430-8.
- Giebels C, Aicher D, Kunihara T, et al. Causes and management of aortic valve regurgitation after aortic valve reimplantation. J Thorac Cardiovasc Surg 2012. [Epub ahead of print].
- Lansac E, Di Centa I, Sleilaty G, et al. An aortic ring: from physiologic reconstruction of the root to a standardized approach for aortic valve repair. J Thorac Cardiovasc Surg 2010;140:S28-35; discussion S45-51.
- Schäfers HJ. Aortic annuloplasty: a new aspect of aortic valve repair. Eur J Cardiothorac Surg 2012;41:1124-5.





