Endoscopic submucosal dissection and endoscopic mucosal resection for early stage esophageal cancer
Introduction
Historically, radical esophagectomy was the standard of care for early esophageal cancer. In the last two decades, endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) have been evolving with promising results. Several studies comparing endoscopic therapy versus surgical resection in patients with TisN0M0 and T1N0M0 esophageal cancer have been recently published. Patients treated with endoscopic therapy had similar median cancer-free survival rates compared with those treated with surgery. Moreover, patients who underwent endoscopic therapy had a significantly lower morbidity rate compared with patients who underwent surgery (1).
EMR and ESD offer non-invasive, less expensive treatments for esophageal cancer limited to the mucosa and without lymph nodes metastasis (2). In this article, we will discuss endoscopic management options for early esophageal cancer.
Endoscopic assessment of early esophageal cancer
Patient selection after extensive and accurate diagnosis and staging is crucial before commitment to endoscopic therapy. An upper endoscopy with multiple mucosal biopsies can diagnose EsC with sensitivity up to 96% (3). Adding brush cytology in a structured esophageal segment can increase the diagnostic accuracy for esophageal cancer to 98.8% (4). Meanwhile, other non-tissue based measures such as chromoendoscopy, narrow band imaging (NBI), confocal endoscopy, spectroscopy, magnification endoscopy, endoscopic ultrasonography (EUS) and other advanced endoscopic imaging techniques are needed to detect the extent and depth of the esophageal cancer. They provide vital information in diagnosing early esophageal cancer as well as guiding the appropriate therapy.
The precise staging of esophageal cancer is crucial for endoscopic therapy qualification.
Depth of tumor invasion, recognition of tumor margins and evaluation of lymph node involvement are essential to determine the feasibility and choice of endoscopic management. Below are some techniques which can aid in selection of lesions amenable to endoscopic treatment.
Chromoendoscopy
Macroscopic features of esophageal cancer can be identified by traditional white-light endoscopies, such as nodules, ulcers or strictures. However, some early esophageal cancers, particularly in high-grade dysplasia, appear macroscopically normal.
Some dyes are applied under white-light endoscopy. Stains used have three major mechanisms, absorptive stains, contrast stains, and reactive stains. Absorptive stains have an affinity for some mucosal elements, including Lugol’s iodine, methylene blue, toluidine blue, and crystal violet (gentian violet). Lugol’s iodine is a solution of elemental iodine and potassium iodide in water. When sprayed onto the surface of the esophageal mucosa, the iodine acts upon the starch of normal squamous epithelium and stain them into black, dark brown, or green-brown after a few minutes. The carcinoma and precancerous lesions (CAPs) which lack starch remain unstained or lightly stained (5). Lugol’s iodine has been the chromoendoscopy agent of choice for evaluation of early esophageal cell carcinoma (6). Methylene blue (MB) is another chromoendoscopy agent that is absorbed by enteric epithelium but not by squamous or gastric epithelium. This selectivity toward enteric epithelium makes it an ideal agent for staining Barrett’s esophagus and highlighting dysplasia in a background of esophageal squamous mucosa. Crystal violet has similar properties to methylene blue (5). Toluidine blue is a basic dye that stains cellular nuclei. It stains malignant tissues, which have an increased DNA synthesis and a high nuclear-to-cytoplasmic ratio, into blue. Toluidine blue is beneficial for both squamous esophageal cancers and esophageal columnar epithelium of Barrett’s esophagus. Unlike vital stains, contrast stains such as indigo carmine are nonabsorbable by the tissue. However, it adds value in highlighting mucosal irregularities (5).
Endoscopy staining with different dyes or combinations can make the presence and extent of esophageal lesions clearer. Chromoendoscopy has a critical role in identifying the borders of the early lesions prior to EMR and ESD.
NBI/FICE/i-scan and magnifying endoscopy
Various new technologies have been applied to better delineate esophageal mucosa. Unlike traditional white-light endoscopy with wave-length ranging from approximately 400 to 800 nm for illumination, narrow-banding imaging (NBI) is a technique where narrow bandwidths of blue and green light are used. The depth of light penetration into the tissue depends on its wavelength. The narrow band light used in NBI preferentially enhances blue light, which penetrates superficially and highlights the superficial capillary network and mucosal pit patterns. The combination of NBI with magnifying or high-resolution endoscopy technology allows visualization of minute structures of the mucosa and fine vascular network (7) (Figure 1). This leads to the recognition of the intrapapillary capillary loops (IPCLs) pattern within the squamous mucosa which results in higher detection of early esophageal carcinoma. There are reports indicating that NBI plus magnifying endoscopy will improve the detection of specialized columnar epithelium and dysplastic epithelium in Barrett’s esophagus. It has high sensitivity and high negative predictive value for detecting superficial esophageal SCC and produces results comparable to those obtained with Lugol’s chromoendoscopy. The other two similar techniques to NBI which have been used in endoscopes are Fuji Intelligent Chromoendoscopy (FICE) (manufactured by Fujinon) and i-SCAN (manufactured by Pentax). Both techniques utilize optical filters or electronic methods to highlight the details of surface patterns and vascular structures. They have all been referred to as virtual chromoendoscopy and have similar diagnostic value for early esophageal cancer. Virtual chromoendoscopy is easier to use, negates the time used for spraying dye and in addition, it can be applied on and off by the click of a button in the endoscope.
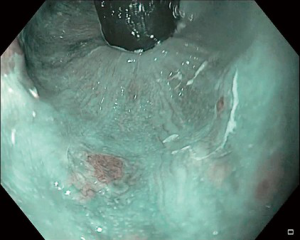
Endoscopic ultrasonography (EUS)
Since EUS was first introduced in the early 1980s, it has been evolving into a valuable diagnostic and therapeutic tool. EUS utilizes echo waves to visualize the histological layers of the esophagus and surrounding tissues. EUS is more sensitive than other imaging modalities in evaluating the depth of invasion of the local tumor and the regional lymph nodes (8). Accuracy for local tumor staging reaches 90% in superficial and partially obstructing esophageal cancers. In a meta-analysis, EUS had a sensitivity of 81.6% and specificity of 99.4% in diagnosing T1. EUS had a pooled sensitivity of 92.4% and specificity of 97.4% in diagnosing T4 lesions. Fine needle aspiration (FNA), increased the sensitivity of EUS to diagnose N stage from 84.7% to 96.7% (9).
EUS using high-frequency ultrasound probes is more accurate than conventional EUS for evaluating the depth of invasion of early esophageal cancer. High-frequency ultrasound probes can accurately detect the depth of invasion in 70–88% of intramucosal cancer and in 83%-94% of submucosal cancer. However, the sensitivity of high-frequency ultrasound probes for the diagnosis of submucosal invasive cancer was relatively low (10,11).
Some reports suggest that the low sensitivity of EUS staging of early-stage esophageal cancers results in under or over treatment of a significant number of patients (12).
The major limitation of EUS is that it is operator dependent and require certain expertise and training to reach proper skills for staging. In addition, EUS is less sensitive in diagnosing GEJ tumors (12). Finally, esophageal cancer with associated stricture could limit the accuracy of EUS due to inability to advance the EUS scope and possible increased risk of perforation.
Local nodes larger than 1 cm with a hypoechogenic round shape are typical for malignant nodes. The sensitivity of EUS in detecting the malignant features of local lymph nodes is 80% (13). EUS guided FNA can prove to be a minimally invasive, safe method to obtain cytology specimens for staging. Adding FNA can improve accuracy up to 92–98% (8,14). Overall, EUS staging for precancerous lesions within Barrett’s esophagus may not yield sufficient information to differentiate mucosal from submucosal invasion but it is helpful in ruling out lymph node metastasis.
Other novel endoscopic techniques available for early esophageal diagnosis
Confocal laser endomicroscopy (CLE)
CLE is an imaging technique which illuminates tissue with a low-power laser allowing a microscopic view of the surface epithelium. The technology requires a contrast injection such as fluorescein. Contrast material diffuses through the capillary to the extracellular matrix with subsequent detection of reflected fluorescent light from the tissue by the laser beam. CLE is capable of obtaining very high magnification and resolution images of the mucosal layer of the GI tract (14). A CLE system could be a through-the-scope based probe (Probe-base CLE, pCLE) or dedicated endoscopy with integrated CLE systems (Endoscope-base CLE, eCLE). Some reports demonstrated that pCLE could visualize tissues at the cellular and subcellular levels with a magnification of 1,000 times, enabling a real-time “optical biopsy” diagnosis of suspicious lesions. CLE is a valid method to differentiate neoplasms from non-neoplasms in BE accurately (15-17). In a meta-analysis by Xiong et al., the sensitivity and specificity of CLE in detecting neoplasia within Barrett’s esophagus was 89% and 83% respectively (18).
Optical coherence tomography (OCT)
OCT can be thought of as a technique analogous to ultrasonography. But unlike EUS, which uses sound waves scattering to produce images, OCT uses infrared light from a laser and optical scattering to create a 2-dimensional image, based on differences in tissue composition. By using a method called interferometry, OCT is capable of measuring interference patterns of a wide-field tissue. The depth-dependent tissue microstructure information can be derived by transforming interference patterns into images in real time with signal-processing algorithms. OCT can differentiate between normal squamous, Barrett’s and gastric mucosa. Volumetric laser endomicroscopy (VLE) is novel balloon-based OCT imaging technique. With this system, the optical components of the catheter are positioned within the esophageal lumen via a balloon-centered probe placed over a guide wire under endoscopic control. The entire portion of the esophageal mucosa in contact with the balloon is scanned in a circumferential and helical manner when the balloon is inflated. VLE can provide a 6-cm long circumferential volumetric scan of the subsurface esophageal wall layers up to 3 mm deep with near microscopic resolution in 96 seconds (19,20) (Figure 2).
Other novel imaging modalities which are beyond the scope of this discussion are fluorescence endoscope, autofluorescence imaging and trimodal imaging (21,22).
Section B: EMR
Background
EMR is a minimally invasive endoscopic technique for directed removal of superficial gastrointestinal benign or early malignant lesions. EMR and ESD have an advantage over ablation techniques in providing enough tissue for adequate histological staging (16).
The basic technique of EMR is cutting and removal of lesions by a through-the-scope snare with or without cautery. Since the majority of early esophageal cancer lesions are flat, it is challenging to trap the lesion into the snare properly. Some auxiliary techniques are developed to handle flat lesions. These techniques include the double-channel endoscope, submucosal injection, cap and ligation assisted EMR.
EMR has a 98.8% complete eradication rate in patients with Barrett’s esophagus without high-risk characteristics (submucosal invasion, poorly differentiated tumors, or evidence of lymphatic or vascular invasion). In patients who had high-risk characteristics, the reported rate was 80.6%. Recurrence rates for both cancer and high-grade dysplasia were 1.4% (17). A 90% sustained remission rate of Barrett’s esophagus-associated neoplasia and intestinal metaplasia was reported in Europe, which was achieved by the combination of initial EMR and subsequent circumferential radiofrequency ablation at least six weeks later (23). As for squamous cell cancers in Asia, in a meta-analysis, the reported en bloc resection rate was 49.3%, and the recurrence rate was 11.5% (17).
EMR successfully eradicates 91% to 98% of T1a cancer (24,25). EMR is considered a relatively safe technique, with complications including bleeding (10%) (24,26), perforation (3%) (24,26) and stricture formation. The risk of stricture formation is proportionate to the size and circumference of the lesion, with up to a 37% risk. Endoscopic dilation successfully manages the majority of strictures (27).
EMR is the preferred technique for nodular lesions in Barrett’s esophagus (25). Combination EMR with radiofrequency ablation has been described in dysplastic Barrett’s esophagus with good results. In a recent meta-analysis, Desai et al. compared patients with Barrett’s esophagus-related high-grade dysplasia and/or intra-mucosal cancer who underwent standard EMR to the patient underwent EMR followed by RFA. The result showed that both techniques had equal eradication rate. However, standard EMR had a higher incidence of bleeding, perforation and stricture formation (28).
Technique for performing EMR:
Injection-assisted EMR
In this technique, injecting solution into the submucosal space beneath the lesion can create a safety cushion. The lesion is then lifted for a snare to cut. The water cushion under the lesion facilitates the capture by snare and minimize mechanical and cautery damage to deeper layers. The submucosal injection is accomplished by injecting saline solution via a needle through the endoscopic channel. Normal saline solution is often used for submucosal injection. However, a cushion made with normal saline solution often dissipates within minutes. Various agents including hyaluronic acid (HA), hydroxypropyl methylcellulose (HPMC), succinylated gelatin, glycerol, and a fibrinogen solution are added for increased cushioning time (29-31). There are currently no specially approved submucosal injection solutions for EMR by the U.S. Food and Drug Administration. However, an approved 0.4% solution of HA in Japan (MucoUp; Johnson & John- son, Tokyo, Japan) demonstrates sustained effect of mucosal lifting and reduced injecting volume (30). Dilute epinephrine (1:100,000–1:200,000) is another agent added into the submucosal injection solution. It had potential benefits of reduced bleeding and sustained submucosal cushion, by decreasing blood flow and subsequently delaying absorption of the fluid. Intraprocedural muscularis propria injury and perforation are easily noticed by staining. The volume of submucosal injection depends on the size of lesion and type of solution. Repeat injections may be required for complete removal.
Ligation-assisted EMR (EMR-L)
Ligation-assisted EMR uses a band ligation device to resect the targeted lesions. Before the procedure, the band ligation device is attached to the tip of the endoscope with a releasing wire through the channel. The endoscope with the ligation device is then wheeled to approach the targeted lesion. When the banding cap of the ligation device is positioned over the targeted lesion, suction is applied to retract the lesion into the cap. The band is then released to ligate the lesion, creating a pseudopolyp. Submucosal injection can be used before suctioning to facilitate creating the pseudopolyp. Once ligated, the target lesion can be removed by electrocautery snare above or below the band. For large lesions, the procedure can be repeatedly applied until complete resection. Multiband mucosectomy (MBM) is a device that uses a modified variceal band ligator without submucosal lifting. The single-use Duette Multi-Band Mucosectomy Kit (Cook Medical, Winston Salem, NC, USA) is one of the ligation devices used for MBM. It consists of six rubber bands on a transparent cap (inner diameter 9 mm), releasing wires attached to a specially designed releasing handle, and a 7-Fr hexagonal braided polypectomy snare, which can be reused for multiple resections (Figure 3). Although both are highly effective and safe, MBM is faster and cheaper than the cap-assisted EMR (32). In recent data, the complete resection rate of MBM was 92.3% with a low acute bleeding complication rate of 7.6%. Delayed bleeding and stenosis complication rates of MBM in this research were both 1.9% (33). A 2.4% local recurrence rate of MBM was reported in a long-term follow-up research of early esophageal squamous cell neoplasia treatment (34).
Cap-assisted EMR (EMR-C)
In cap-assisted EMR, a transparent cap is first affixed to the tip of the endoscope. A specially designed crescent-shaped electrocautery snare is then opened and positioned on the internal circumferential ridge at the tip of the cap. After being located over the target lesion, suctioning the lesion into the cap is attempted. Once the lesion is retracted completely into the cap, electrocautery snare close, capture and resect the lesion. A submucosal injection is often needed to facilitate suction and provide a cushion. Caps are soft or hard clear plastic, cylindrical and available with a flat circular (straight)—or oval (oblique)—shaped tip. Like ligation-assisted EMR, due to diameters of the cap ranging from 12.9 to 18 mm, larger lesions can only be removed by piece-meal resection, which may increase the risk of residual neoplasia and potential metastasis. Conio et al. reported a 91% complete eradication of neoplasia and metaplasia rate in BE by circumferential cap-assisted EMR. The median follow-up period was 18.4 months. This method had a high stenosis rate of 40% which was treated with dilations and covered stent endoscopically (35).
Section C: enodscopic submucosal dissection (ESD)
Background
ESD was firstly introduced in 1988 by Japanese endoscopists for early superficial gastric cancer treatment and biopsy (36). Over the ensuing decades, many needle knives were developed and ESD evolved into an advanced endoscopic procedure which can provide en bloc resection of large GI mucosal and submucosal lesions. In a recent systemic review and meta-analysis of ESD in gastroesophageal junction lesions, en bloc resection and complete resection was achieved in 98.6% and 87.0% of lesions respectively. When curative resections are achieved, no local recurrence and distant metastasis occurred (37).
Generally, ESD indications for esophageal cancer are stricter than for gastric cancer. ESD can only be considered in patients without lymphovascular invasion. The lymphovascular invasion is mainly based on the tumor’s depth, which could be evaluated by a pre-procedure assessment on the macroscopic type, magnifying narrow-band imaging endoscopy for squamous cell carcinoma and high-frequency probe-based EUS. Although the actual depth of invasion is unknown until pathologic analysis, ESD is beneficial in providing en bloc specimen, such that noncurative resections can be more easily detected and referred for further oncologic surgery (37).
The Japanese Esophageal Society issued absolute indications for esophageal cancer ESD which are intramucosal cancers involving the epithelium and lamina propria occupying <2/3 of the lumen of the esophagus along with relative indications, which are esophageal cancer involving the muscularis mucosa or <200 µm invasion of the submucosa (38). In western society, a majority of early esophageal cancer is adenocarcinomas originating from BE. The reasonable indication for ESD in this category is high grade dysplasia or intramucosal adenocarcinoma. The current guidelines do not set any limitations for performing ESD based on tumor length (38).
This recommendation was based on incidence of lymph metastasis in T1a EAC of 0–2.6% (39). In comparison, the incidence of lymph node metastasis is 0–33% in T1b SM1 tumors (tumors extending to the upper third of the submucosa) and up to 60% for T1b SM2-3 tumors (tumors extending to the middle and lower third of the submucosa) (40). Giving the high risk of lymph node metastasis in T1b SM2-3 tumors, these lesions should be managed with surgery. ESD is favored over EMR for lesions larger than 15 mm, lesion with poor lifting and to better assess depth of invasion if submucosal invasion was to be suspected (41).
Technique of esophageal ESD procedure
ESD is performed with a standard, single accessory-channel endoscope. Carbon dioxide is used for insufflation. Special equipment necessary for ESD are a transparent cap, submucosal injection needle and solutions, ESD knives, coagulation devices, and endoclips. Typical ESD is accomplished in a stepwise manner including marking the lesion, incision and submucosal dissection with simultaneous hemostasis.
Marking the lesion
Absolute delineation and definition of the border of esophageal neoplasms is crucial. Chromoendoscopy using several dyes, or NBI with magnification are often used for pre-procedural assessment. Once the margins of the lesion are fully visualized, an argon plasma coagulation (APC) or ESD knife using soft coagulation current can be applied to mark the resection borders with dots around the lesion at least 5 mm away from the margin. The marked resection border is easily recognized during circumferential incising, especially when the submucosal injection distorts the appearance of the lesion.
Creating a submucosal fluid cushion
After the resection borders are marked, fluids can be injected beneath the mucosa by a submucosal injection needle through the endoscopic channel to create a cushion. Normal saline solution is safe and economical solution for injection but does not provide long-lasting cushion. Hypertonic saline solution and dextrose may cause local tissue damage (42). Sodium hyaluronate 0.4% (MucoUp; Johnson and Johnson, Tokyo, Japan) is widely used in Asian centers of expertise. In non-Asian countries, 0.4% hydroxypropyl methylcellulose is widely accepted and it is relatively inexpensive. Several dyes, typically indigo carmine, can be added into the solution to help differentiate tissue planes. The addition of epinephrine is somewhat controversial. It can help reduce procedural bleeding but was reported to increase the risk of gastric ischemia and myocardial infarction (43,44). Recently, new submucosal injection solutions with audodissection properties are under evaluation (45,46).
Circumferential incising
Circumferential incision is made along the dots marked around the lesion. The incisions between marking dots connect to form a circle which separates the lesion from normal mucosa (Figure 4). For complete and en bloc resection, it is recommended that the circumferential incision should be started outside the dots rather than inside. There are several specially designed commercial ESD knives for cutting such as dual knife, IT knife, IT nano knife, hook knife and flex knife (47). Most ESD knives can be used in multiple steps of ESD. The utility of these knives depend on the operator’s personal experiences and preference.
Dissecting the submucosal layer beneath the lesion
Submucosal dissection is a challenging and time-consuming step. The entire lesion is stripped or peeled from the muscularis propria by ESD knives in the submucosal space. During this step, the submucosal injection needle and ESD knives are used interchangeably to lift the lesion and dissect the submucosal tissue. HybridKnife is an ESD knife specially designed for both purposes. It has a fine capillary in the core of the 5-mm cutting knife, which can serve as a 120-mm water jet when connected to a foot pedal and computerized jet lavage unit (ERBEJET 2 system; ERBE USA). The HybridKnife system allows the operator to perform the submucosal injection and dissection without changing the device. Water is injected with proper pressure by ultrafine water jets that can penetrate the mucosa and submucosal space to lift and provide a cushion without needle punctuation.
Minor oozing from small blood vessels can be treated with current coagulation flow directly delivered by ESD knives from Electrosurgical units (ESU). For more significant bleeding, hemostatic forceps or a coagulation grasper can be used with a relative electrosurgical current to stop the bleeding. Several newer ESUs provide multiple pre-settings and functionality that facilitate safe and effective ESD. One of the commonly used units is ERBE VIO300D unit (ERBE USA), it has a SOFT COAG mode which provides a continuous current of less than 190Vp. SOFT COAG mode is very useful for vessel coagulation with hemostatic forceps (i.e., Coagrasper). Its other modes like DRY CUT and ENDOCUT also provide different cutting and coagulation effects by using different duty cycle and electrosurgical waveforms. It is recommended to reduce intraprocedural bleeding by prophylactic coagulation with hemostatic forceps to handle larger non-bleeding submucosal vessels during the dissection (Figure 5).
Treatment of artificial ulcer after ESD
After accomplishing the dissection, the lesion can be removed by forceps, transparent cap or basket and processed for histological evaluation. An artificial ulcer is then created with muscularis propria. It is important to inspect the ulcer bed for micro-perforation or exposed blood vessels. Hemoclips are commonly used for closure of perforations and possible bleeding vessels during the inspection. Liquid antacids such as sucralfate are applied by spraying the surface of the ulcer through the endoscope via a catheter or injection needle for facilitating healing. Intravenous administration of proton pump inhibitors (PPI) in the first several days after the procedure followed by oral administration for several weeks is recommend standard of care treatment for the post ESD ulcer.
Specimen processing and histological evaluation
Proper specimen handling is crucial to provide a consistent and accurate diagnosis. Several factors such as maintenance of proper orientation, meticulous macroscopic examination, accurate mapping of the lesion, and appropriate morphologic diagnosis are the main concerns. The specimen needs to be pinned against a plate peripherally by stainless-steel pins and then immersed in formaldehyde immediately to preserve the tissue size, shape, and orientation. Lugol’s solution staining can be used again for macroscopic delineation of the lesion. After immersion in formalin overnight, the specimen is measured in 2 dimensions according to the location and closest margin of the lesion. Then the specimen is sectioned at 2–3 mm (optimally 2.5 mm but no less than 2 mm) parallel to the oral/anal plane or accommodate to the interested margin. A picture with all of these annotations and ruler in place is recommended before sectioning. The maintenance of orientation is crucial in the following; slicing, histological analysis and reporting. Specimens are always entirely submitted in sequential order for histopathologic evaluation. Factors relevant to prognosis and further treatment decisions including histologic type, the size of the lesion, depth of invasion, association conditions (ulcer/scar), lymphovascular/venous invasion, and cut margin status (horizontal and vertical) should always be carefully evaluated and reported.
Outcomes of ESD
Isomoto et al. reported en bloc resection rates of 90–100% for esophageal SCC and 97–100% for esophageal AC using ESD. Curative resection rates were 88–99.1% for SCC vs. 79–97% for AC (48). Probst et al. studied the outcome of 24 patients with esophageal SCC and 87 patients with esophageal AC who underwent ESD. The en bloc resection rates were 100% for SCC vs. 95.4% for AC. R0 resection rates were 91.7% for SCC vs. 83.9% for AC. R0 resection was higher in Barrett’s lesion ≤ M3 (90%) compared to lesions > M3 (70.4%). The curative resection rates were 45.8 % for SCC vs. 72.4 % for AC. Only AC was observed with local recurrence of 2.4% (49).
In a recent meta-analysis from Asian populations comparing ESD to EMR, ESD had significantly higher curative resection rates and lower local recurrence rate than EMR, particularly in lesions less than 2cm. However, operative time and perforation rate were significantly higher in the ESD group compared to EMR group. Risk of bleeding or stricture were equal between the two groups (17).
Complications of esophageal endoscopic resection include pain, intra-procedural and delayed bleeding, stricture, perforation with subsequent potential pneumothorax, hemopneumothorax and pneumomediastinum. The most frequent complication of ESD is intra-procedural bleeding. A recent review estimated complication rates after ESD for esophageal cancer to be around 2.6–10% perforation rate and 0.7–5.2% bleeding rate (48).
Most perforations can be identified during the procedure and managed by clip closure. Delayed perforation due to artificial ulcer after esophageal ESD is rare but may result in severe or even life-threatening conditions like mediastinal emphysema or mediastinitis (50). Early recognition and subsequent surgical management are essential. Minimal subcutaneous emphysema may result due to escaped air from esophageal muscles fibers, and can be treated with conservative management. CO2 is highly recommended for insufflation during esophageal ESD. Over-the-scope clip (OTSC) system (Ovesco, Germany), which is delivered over the scope can provide better tissue capture compared to conventional clips (51).
Strictures are another frequently mentioned complication post-ESD. Due to the tube-like structure, the esophagus has the highest rates of stricture complication compared to other areas in the GI tract. Post ESD esophageal stricture is defined as narrowing due to esophageal ESD procedure through which a standard endoscope can’t pass. Circumference and length of resection area are the main risk factors. Esophageal stricture occurs in patients who undergo more than a 75% circumference ESD resection of the esophagus. Multiple management aimed at preventing and treating post-ESD esophageal stricture can be applied, which include multiple sessions of endoscopic balloon dilatation (EBD), local injection of steroids (triamcinolone, betamethasone), implantation of a temporal esophageal stent, systemic steroid (prednisolone) administration, and systemic N-acetylcysteine administration. Some new methods are under investigation in animal models at the moment, such as endoscopic injection of autologous oral mucosal epithelial or adipose tissue-derived stromal cells and endoscopic transplantation of tissue-engineered cell sheet of autologous oral mucosal epithelial cells.
We typically recommend follow up endoscopy in 3 months for surveillance after performing ESD or EMR. Although EMR and ESD can achieve complete resection of early esophageal adenocarcinoma, it is difficult to completely eradicate the surrounding Barrett’s esophagus with ESD or EMR alone. Radiofrequency ablation of the residual Barrett’s tissue is recommended after ESD or EMR to decrease the risk of recurrent tumor (52).
Conclusions
Endoscopic resection of early esophageal cancer is a feasible and safe treatment modality for esophageal cancer. EMR and ESD are acceptable treatment modalities for early esophageal cancer. ESD requires technical expertise but is associated with higher rates of en bloc, R0, and curative resections in addition to lower recurrence rates compared to EMR. Sufficient training is crucial to ensure safe and high-quality resections.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Das A, Singh V, Fleischer DE, et al. A comparison of endoscopic treatment and surgery in early esophageal cancer: an analysis of surveillance epidemiology and end results data. Am J Gastroenterol 2008;103:1340-5. [Crossref] [PubMed]
- Kruszewski WJ. Endoscopic methods in the treatment of early-stage esophageal cancer. Wideochir Inne Tech Maloinwazyjne 2014;9:125-30. [Crossref] [PubMed]
- ASGE Standards of Practice Committee, Evans JA, Early DS, et al. The role of endoscopy in the assessment and treatment of esophageal cancer. Gastrointest Endosc 2013;77:328-34. [Crossref] [PubMed]
- Zargar SA, Khuroo MS, Jan GM, et al. Prospective comparison of the value of brushings before and after biopsy in the endoscopic diagnosis of gastroesophageal malignancy. Acta Cytol 1991;35:549-52. [PubMed]
- Canto MI. Staining in gastrointestinal endoscopy: the basics. Endoscopy 1999;31:479-86. [Crossref] [PubMed]
- Ragunath K, Krasner N, Raman VS, et al. A randomized, prospective cross-over trial comparing methylene blue-directed biopsy and conventional random biopsy for detecting intestinal metaplasia and dysplasia in Barrett's esophagus. Endoscopy 2003;35:998-1003. [Crossref] [PubMed]
- Mochizuki Y, Saito Y, Kobori A, et al. Magnified endoscopy combined with narrow band imaging of minimal superficial esophageal neoplasia-indicators to differentiate intraepithelial neoplasias. J Gastrointest Cancer 2012;43:599-606. [Crossref] [PubMed]
- Wiersema MJ, Vilmann P, Giovannini M, et al. Endosonography-guided fine-needle aspiration biopsy: diagnostic accuracy and complication assessment. Gastroenterology 1997;112:1087-95. [Crossref] [PubMed]
- Puli SR, Reddy JB, Bechtold ML, et al. Staging accuracy of esophageal cancer by endoscopic ultrasound: a meta-analysis and systematic review. World J Gastroenterol 2008;14:1479-90. [Crossref] [PubMed]
- Yoshinaga S, Oda I, Nonaka S, et al. Endoscopic ultrasound using ultrasound probes for the diagnosis of early esophageal and gastric cancers. World J Gastrointest Endosc 2012;4:218-26. [Crossref] [PubMed]
- Thomas T, Gilbert D, Kaye PV, et al. High-resolution endoscopy and endoscopic ultrasound for evaluation of early neoplasia in Barrett's esophagus. Surg Endosc 2010;24:1110-6. [Crossref] [PubMed]
- Bergeron EJ, Lin J, Chang AC, et al. Endoscopic ultrasound is inadequate to determine which T1/T2 esophageal tumors are candidates for endoluminal therapies. J Thorac Cardiovasc Surg 2014;147:765-71: Discussion 771-3.
- Bhutani MS, Hawes RH, Hoffman BJ. A comparison of the accuracy of echo features during endoscopic ultrasound (EUS) and EUS-guided fine-needle aspiration for diagnosis of malignant lymph node invasion. Gastrointest Endosc 1997;45:474-9. [Crossref] [PubMed]
- Eloubeidi MA, Wallace MB, Reed CE, et al. The utility of EUS and EUS-guided fine needle aspiration in detecting celiac lymph node metastasis in patients with esophageal cancer: a single-center experience. Gastrointest Endosc 2001;54:714-9. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Ohja B, et al. The accuracy of endoscopic ultrasonography with fine-needle aspiration, integrated positron emission tomography with computed tomography, and computed tomography in restaging patients with esophageal cancer after neoadjuvant chemoradiotherapy. J Thorac Cardiovasc Surg 2005;129:1232-41. [Crossref] [PubMed]
- Moss A, Bourke MJ, Hourigan LF, et al. Endoscopic resection for Barrett's high-grade dysplasia and early esophageal adenocarcinoma: an essential staging procedure with long-term therapeutic benefit. Am J Gastroenterol 2010;105:1276-83. [Crossref] [PubMed]
- Guo HM, Zhang XQ, Chen M, et al. Endoscopic submucosal dissection vs endoscopic mucosal resection for superficial esophageal cancer. World J Gastroenterol 2014;20:5540-7. [Crossref] [PubMed]
- Xiong YQ, Ma SJ, Zhou JH, et al. A meta-analysis of confocal laser endomicroscopy for the detection of neoplasia in patients with Barrett's esophagus. J Gastroenterol Hepatol 2016;31:1102-10. [Crossref] [PubMed]
- Swager A, Boerwinkel DF, de Bruin DM, et al. Volumetric laser endomicroscopy in Barrett's esophagus: a feasibility study on histological correlation. Dis Esophagus 2016;29:505-12. [Crossref] [PubMed]
- Lightdale CJ. Optical coherence tomography in Barrett's esophagus. Gastrointest Endosc Clin N Am 2013;23:549-63. [Crossref] [PubMed]
- Boerwinkel DF, Di Pietro M, Liu X, et al. Endoscopic TriModal imaging and biomarkers for neoplasia conjoined: a feasibility study in Barrett's esophagus. Dis Esophagus 2014;27:435-43. [Crossref] [PubMed]
- Sturm MB, Wang TD. Emerging optical methods for surveillance of Barrett's oesophagus. Gut 2015;64:1816-23. [Crossref] [PubMed]
- Phoa KN, Pouw RE, van Vilsteren FG, et al. Remission of Barrett's esophagus with early neoplasia 5 years after radiofrequency ablation with endoscopic resection: a Netherlands cohort study. Gastroenterology 2013;145:96-104. [Crossref] [PubMed]
- Pech O, Behrens A, May A, et al. Long-term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett's oesophagus. Gut 2008;57:1200-6. [Crossref] [PubMed]
- Ell C, May A, Pech O, et al. Curative endoscopic resection of early esophageal adenocarcinomas (Barrett's cancer). Gastrointest Endosc 2007;65:3-10. [Crossref] [PubMed]
- Pouw RE, van Vilsteren FG, Peters FP, et al. Randomized trial on endoscopic resection-cap versus multiband mucosectomy for piecemeal endoscopic resection of early Barrett's neoplasia. Gastrointest Endosc 2011;74:35-43. [Crossref] [PubMed]
- Chennat J, Konda VJ, Ross AS, et al. Complete Barrett's eradication endoscopic mucosal resection: an effective treatment modality for high-grade dysplasia and intramucosal carcinoma--an American single-center experience. Am J Gastroenterol 2009;104:2684-92. [Crossref] [PubMed]
- Desai M, Saligram S, Gupta N, et al. Efficacy and safety outcomes of multimodal endoscopic eradication therapy in Barrett's esophagus-related neoplasia: a systematic review and pooled analysis. Gastrointest Endosc 2017;85:482-95.e4. [Crossref] [PubMed]
- Fujishiro M, Yahagi N, Kashimura K, et al. Comparison of various submucosal injection solutions for maintaining mucosal elevation during endoscopic mucosal resection. Endoscopy 2004;36:579-83. [Crossref] [PubMed]
- Yamamoto H, Yahagi N, Oyama T, et al. Usefulness and safety of 0.4% sodium hyaluronate solution as a submucosal fluid "cushion" in endoscopic resection for gastric neoplasms: a prospective multicenter trial. Gastrointest Endosc 2008;67:830-9. [Crossref] [PubMed]
- Arantes V, Albuquerque W, Benfica E, et al. Submucosal injection of 0.4% hydroxypropyl methylcellulose facilitates endoscopic mucosal resection of early gastrointestinal tumors. J Clin Gastroenterol 2010;44:615-9. [Crossref] [PubMed]
- Zhang YM, Boerwinkel DF, Qin X, et al. A randomized trial comparing multiband mucosectomy and cap-assisted endoscopic resection for endoscopic piecemeal resection of early squamous neoplasia of the esophagus. Endoscopy 2016;48:330-8. [PubMed]
- Jin XF, Sun QY, Chai TH, et al. Clinical value of multiband mucosectomy for the treatment of squamous intraepithelial neoplasia of the esophagus. J Gastroenterol Hepatol 2013;28:650-5. [Crossref] [PubMed]
- Wang Z, Lu H, Wu L, et al. Long-term outcomes of endoscopic multiband mucosectomy for early esophageal squamous cell neoplasia: a retrospective, single-center study. Gastrointest Endosc 2016;84:893-9. [Crossref] [PubMed]
- Conio M, Fisher DA, Blanchi S, et al. One-step circumferential endoscopic mucosal cap resection of Barrett's esophagus with early neoplasia. Clin Res Hepatol Gastroenterol 2014;38:81-91. [Crossref] [PubMed]
- Hirao M, Masuda K, Asanuma T, et al. Endoscopic resection of early gastric cancer and other tumors with local injection of hypertonic saline-epinephrine. Gastrointest Endosc 1988;34:264-9. [Crossref] [PubMed]
- Park CH, Kim EH, Kim HY, et al. Clinical outcomes of endoscopic submucosal dissection for early stage esophagogastric junction cancer: a systematic review and meta-analysis. Dig Liver Dis 2015;47:37-44. [Crossref] [PubMed]
- Ono S, Fujishiro M, Koike K. Endoscopic submucosal dissection for superficial esophageal neoplasms. World J Gastrointest Endosc 2012;4:162-6. [Crossref] [PubMed]
- Bhatt A, Abe S, Kumaravel A, et al. Indications and Techniques for Endoscopic Submucosal Dissection. Am J Gastroenterol 2015;110:784-91. [Crossref] [PubMed]
- Ancona E, Rampado S, Cassaro M, et al. Prediction of lymph node status in superficial esophageal carcinoma. Ann Surg Oncol 2008;15:3278-88. [Crossref] [PubMed]
- Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2015;47:829-54. [Crossref] [PubMed]
- Fujishiro M, Yahagi N, Kashimura K, et al. Tissue damage of different submucosal injection solutions for EMR. Gastrointest Endosc 2005;62:933-42. [Crossref] [PubMed]
- Probst A, Maerkl B, Bittinger M, et al. Gastric ischemia following endoscopic submucosal dissection of early gastric cancer. Gastric Cancer 2010;13:58-61. [Crossref] [PubMed]
- Kim HH, Park MI, Park SJ, et al. Myocardial infarction thought to be provoked by local epinephrine injection during endoscopic submucosal dissection. J Clin Med Res 2011;3:143-6. [PubMed]
- Sumiyama K, Toyoizumi H, Ohya TR, et al. A double-blind, block-randomized, placebo-controlled trial to identify the chemical assistance effect of mesna submucosal injection for gastric endoscopic submucosal dissection. Gastrointest Endosc 2014;79:756-64. [Crossref] [PubMed]
- Khashab MA, Saxena P, Sharaiha RZ, et al. A novel submucosal gel permits simple and efficient gastric endoscopic submucosal dissection. Gastroenterology 2013;144:505-7. [Crossref] [PubMed]
- Inoue H, Minami H, Kobayashi Y, et al. Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy 2010;42:265-71. [Crossref] [PubMed]
- Isomoto H, Yamaguchi N, Minami H, et al. Management of complications associated with endoscopic submucosal dissection/ endoscopic mucosal resection for esophageal cancer. Dig Endosc 2013;25 Suppl 1:29-38. [Crossref] [PubMed]
- Probst A, Aust D, Markl B, et al. Early esophageal cancer in Europe: endoscopic treatment by endoscopic submucosal dissection. Endoscopy 2015;47:113-21. [PubMed]
- Hanaoka N, Uedo N, Ishihara R, et al. Clinical features and outcomes of delayed perforation after endoscopic submucosal dissection for early gastric cancer. Endoscopy 2010;42:1112-5. [Crossref] [PubMed]
- Nishiyama N, Mori H, Kobara H, et al. Efficacy and safety of over-the-scope clip: including complications after endoscopic submucosal dissection. World J Gastroenterol 2013;19:2752-60. [Crossref] [PubMed]
- Neuhaus H, Terheggen G, Rutz EM, et al. Endoscopic submucosal dissection plus radiofrequency ablation of neoplastic Barrett's esophagus. Endoscopy 2012;44:1105-13. [Crossref] [PubMed]