Technical pitfalls and tips for the valve-in-valve procedure
Introduction
Individuals who require a replacement valve have options of a mechanical valve which is durable but requires lifelong anticoagulation or a bioprosthetic valve without the need for anticoagulation but with limited durability (1). In the last decade, there is a trend for the patients to choose bioprosthetic valves over mechanical valves and hence during their lifetime, a large number of these patients will require reoperation. The risks of a redo procedure are usually higher than those of the first operation. Further, age and comorbidities may preclude many patients from such an operation (2). As an alternative, valve-in-valve (VIV) can be performed in a minimally invasive fashion without the need for cardio-pulmonary bypass within the degenerated surgical heart valves (SHV) and has emerged as an attractive option for these patients (3-6). A SHV provides a perfect circular platform to allow even expansion of the transcatheter heart valves (THV) and, in most cases, a suitable anchor for fixation. The procedure is of shorter duration and can be performed under conscious sedation in the majority of the patients, facilitating early recovery (3-6). Avoidance of operative trauma, reduced blood loss and minimal use of contrast further reduces the risk of complications. Optimal pre-procedural planning with the help of angiography and CT scanning is critical (7,8). In this article, we describe a step-by-step approach to a VIV procedure to optimize outcomes and minimize the risk of operative complications.
Operative technique
Procedural planning
Identification of the SHV
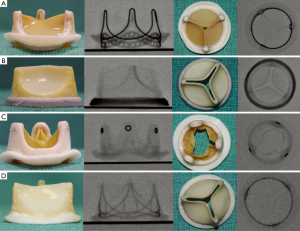
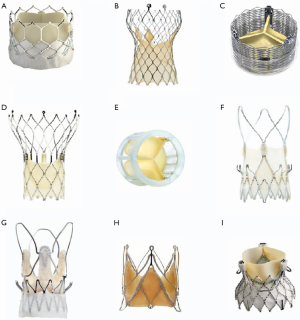
The first step is to confirm the model and size of the SHV (9). This preoperative step is critical. Either a valve card or records from previous operation notes or the manufacturer are the usual sources of this information. If this information is not available, the SHV can be identified by way of its unique fluoroscopic appearance in the majority of the patients (Figure 1) (9). Some stented and most stentless SHVs may not be visible under fluoroscopy and pose a unique challenge in VIV planning. In these situations, ECG-gated CT and echocardiography play important roles in procedural planning.
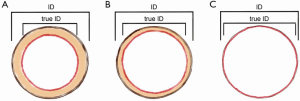
True ID
Manufacturers provide a label or nominal size, which in turn can provide a stent ID. This stent ID, however, can misguide the user in selecting an optimal THV. The stent ID is a measurement of the stent frame without leaflets. The type of leaflets, i.e., porcine leaflets or bovine pericardium, and method of mounting, i.e., inside or outside the stent frame, influences the true ID (Figure 2) (10). For a VIV procedure, the true ID is the measurement of interest. This information can easily be obtained from the VIV App (11).
THV type and size
A THV is selected on the basis of user preference and the anticipated risk of complications, including malposition due to borderline size or an invisible valve, coronary obstruction and the risk of a higher residual gradient due to a smaller true ID (Figure 3). The majority of cases can be treated with either a balloon-expandable SAPIEN or self-expandable CoreValve Evolut R (Evolut R) platform. In certain situations when there will likely be need for repositioning due to unclear landmarks or need for assessment of coronary arteries, a device that can be repositioned and retrieved, such as Lotus valve (Boston Scientific, MN), may be preferred. Size of the THV chosen depends on the oversize needed to fix the valve. Each THV valve has its own sizing algorithm and, for an aortic VIV, an oversize of 1–2 mm is usually sufficient. If the SHV is stenotic with leaflet calcification and thickening or there is evidence of pannus growth, we tend to use an oversize of only 1mm. This is important as it allows complete and even expansion of the THV, which in turn determines function and long-term durability. This information should be confirmed from the VIV App (11).
Fluoroscopic appearance
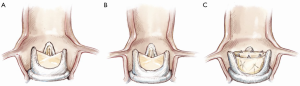
It is important to identify landmarks for optimal deployment of the THV within the SHV to achieve secure fixation and optimal valve function. The aortic annulus is used as a reference line during THV positioning within a native aortic valve. Similarly, the level of the neo-annulus of the SHV is identified by correlating the structure of the SHV to its fluoroscopic appearance (12). This neo-annulus is at the level of the sewing ring of the surgical valve (Figure 4). In some SHVs, there is a marker within the sewing ring which facilitates identification of this level (Figure 1B). Some valves have their stent frame visible and for these it is important to assess the relationship between the inflow and the level of the sewing ring. (Figure 1A,D). In SHVs with no fluoroscopic marker in the frame or sewing ring, this level can be determined during the procedure by the use of pig-tail catheter with multiple contrast injections, balloon valvuloplasty or transesophageal echocardiography (TEE) (Figure 1C) (13).

Access route
The majority of procedures can be performed using a retrograde transfemoral (TF) approach. This is by far the least invasive approach, which can be performed with percutaneous closure and under conscious sedation. Further, most THV devices can be implanted using this approach. For anatomical difficulties resulting from calcification, tortuosity and reduced calibre, the TC approach is not feasible and an alternative route is used. Depending on the THV device used, experience of the team and anatomical factors, alternative routes include trans-subclavian (TS), trans aortic (Tao), trans-apical (TA), trans-caval (TC) and trans-carotid. The TA approach is only feasible with a few devices, including the SAPIEN, Symetis Accurate TA and JenaValve.
Risk of coronary obstruction
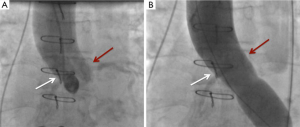
Although the incidence of coronary obstruction is <2%, it is a life-threatening complication and hence this risk of coronary obstruction must be assessed conscientiously before performing a VIV procedure (7,8,14,15). This risk of obstruction is influenced by interaction between the patient’s native anatomy and type of SHV (7,8,15). After a VIV procedure, the SHV leaflets are pushed out towards the coronary ostia, hence risk is higher if the aortic root is narrow (Figure 5A,B). Similarly, where the SHV has pericardial leaflets rather than porcine leaflets, where leaflets are mounted outside the stent frame of a stented SHV and in cases of stentless SHVs, the risk of obstruction is higher. This can be assessed to an extent by angiography, whereby the height of the coronary ostia in relation to the height of the SHV stent and size of the SHV relative to aortic root can be determined. CT scanning may be preferable as the distance between the stent frame and sinus of Valsalva can be measured in cross-section. This is referred to as the virtual THV-coronary (VTC) distance (Figure 5C) (7). If it is >6 mm, the risk is minimal, between 4-6mm is borderline and <4 mm is high-risk for coronary obstruction (7). If the risk is borderline or if there is no surgical option, then bailout planning is essential. This may be in the form of readiness for CPB and coronary artery stenting. Stenting the ostia with part of stent in the sinuses of Valsalva is a means of preventing this complication. The only other option is open-heart surgery with a coronary artery bypass graft with or without removal of the VIV complex.
Preparation
The procedure should be performed in either a hybrid laboratory or a well-equipped catheter laboratory with facilities for partial or complete hemodynamic support. This is especially important in cases where a coronary obstruction is anticipated. Although the need for emergency cardiopulmonary bypass (CPB) is less than 1%, when needed, the speed of with which CPB is commenced determines the outcome. Approaches other than a TF approach are usually performed under general anaesthesia (GA). The patient is positioned supine with arms tucked on either side. For TF and trans-caval approaches, the team is positioned on the right side of the table near the right leg. For the TS and trans-carotid approaches the team is positioned near the left or right shoulder. For the transaortic approach, the team is positioned near the right shoulder. Essential equipment includes catheters necessary for performing the VIV procedure, percutaneous closure devices, coronary guide wires and stents, peripheral stents for access site complications and finally equipment for CPB if complication is anticipated.
Exposition
A TEE probe is inserted if the patient is under a GA. When TEE cannot be performed, a transthoracic probe is used. Attention is paid to the position of the X-ray tube relative to the position of the operating team. Optimal fluoroscopic views are checked before prepping the operative field to ensure a smooth procedure. This also allows the operator to gauge the presence of other radio-opaque shadow overlaps the field of interest, which is not uncommon.
Operation
The TF approach will be described in detail as it is the most commonly used approach for VIV implantation.
Access site preparation
A suitable site is chosen after assessment of the vasculature on CT scan, using 3-Mensio software (Pie medical, Netherlands). Other commercially available softwares can also be used (Circle, Osirix and Horos). Femoral artery puncture is made in the region of femoral head but away from femoral artery bifurcation. Preparation is made for percutaneous closure using two Proglide sutures (Abbott, MN). A 7-French sheath is introduced. On the contralateral side, femoral arterial and venous access is obtained. The arterial access site is used to insert a pig-tail catheter into the aortic root. Using this catheter, a final assessment of the aortic root and coronary ostia is made if necessary. The femoral vein access is used to insert a pacing lead into the right ventricle. We prefer a balloon-tip flexible pacing lead to reduce the risk of perforation. Rapid pacing is utilized during implantation of majority of THV devices.
Deployment view
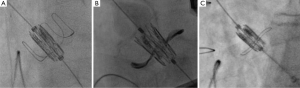
For stented SHVs, a deployment view is obtained by aligning the fluoroscopic markers. Unlike that for the native aortic valve, there is no need of multiple contrast injections (Figure 6). One can use a 2:1 view as described by the Vancouver group if left main coronary obstruction is a possibility. This view facilitates assessment of the left main coronary artery during the procedure.
Reference plane
As discussed earlier, the neo-annular plane is identified and is used as a reference plane for THV crossing and positioning. When the valve is poorly or not visualized, the pig-tail catheter is placed right at the bottom of the aortic root and contrast is injected when needed to facilitate delineation of the neo-annulus.
Valve crossing and sheath insertion
Through the 7-Fr arterial sheath, a JR4 catheter is inserted over a soft J-tip guidewire. The JR4 catheter is advanced into the ascending aorta. Using a straight tip wire, the valve is crossed in retrograde fashion. If the valve crossing is difficult, one can use either an AL1 or AL2 catheter as well as a hydrophilic straight tip wire. Once the valve is crossed, the JR4 or pigtail is advanced in to the left ventricle. This allows placement of a stiffer wire into the left ventricle. Care is taken to avoid ventricular injury and interference with the mitral valve apparatus. One can confirm optimal placement of the wire with TEE when available. We have now prefer a pre-shaped Safari (Boston Scientific) or Confida (Medtronic Inc., MN, USA) wire. In cases of highly stenotic SHVs, we use a much stiffer wire, such as Lunderquist wire (Cook Medical) to facilitate crossing of the THV device across the degenerated SHV. Once the wire is in place, the 7-Fr sheath is removed and a suitable sheath for THV delivery is inserted.
Balloon aortic valvuloplasty
Balloon aortic valvuloplasty (BAV) is performed selectively as it carries risks of leaflet injury. We prefer to perform BAV with a small-sized balloon (16 to 18 Fr) when the SHV is stenotic and when valve crossing is anticipated to be difficult e.g. in the case of tortuous peripheral vessels or a horizontal aorta. Following thorough planning, a balloon is placed over the stiff wire across the SHV. A short duration of rapid pacing is used to perform BAV. The balloon is then removed, ensuring that the wire position in the ventricle remains unchanged. BAV is also useful in assessing the risk of coronary obstruction (Figure 7).
THV preparation and insertion
When the SAPIEN device is used, valve orientation is checked prior to crimping. Similarly, when the Evolut R valve is used, fluoroscopic confirmation of satisfactory loading of the tabs within the system is performed. The delivery system is introduced over the wire. In the case of the SAPIEN valve, the device is aligned within the descending aorta, advanced under fluoroscopic guidance across the arch and is placed just above the SHV. Crossing is performed in the deployment view. This allows the operator to visualize progress of the THV in real time, thus avoiding excessive manipulation of the THV device in a degenerated SHV (Figure 8). The device is placed in the optimal position in relation to the neo-annulus. TEE is used to optimize position of the device in cases where the device is not visible under fluoroscopy.
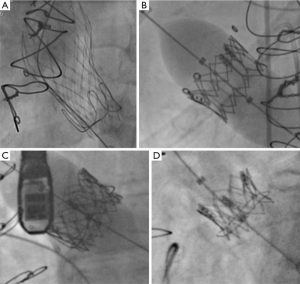
Valve deployment
We like to position the device no lower than 4 mm below the neo-annulus to ensure secure fixation (provided the correct size of THV is chosen) and optimal hemodynamic result. This is especially true when using Evolut R, as a supra-annular device, which will function as an intra-annular valve if placed deeper. It should be possible to achieve this position in most cases with its new feature which allows repositioning. When using the SAPIEN valve, slow deployment ensures optimal positioning, which is critical as it cannot be repositioned or retrieved after deployment (Figure 9).
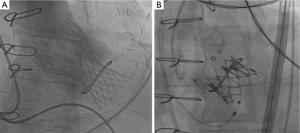
Completion
Once the valve is deployed, the system is withdrawn but the wire is maintained within the left ventricle. An echocardiographic assessment is performed to assess valve position, function and the presence of central or paravalvular leak. If needed, an additional BAV is performed to ensure complete expansion of the THV. Following this, the guidewire and delivery system are withdrawn carefully. Particular care is needed to not dislodge the newly implanted THV. Angiography may be performed thereafter.
Closure
The sheath is removed and the Proglide sutures are tied. There are various techniques used to achieve vascular closure. We prefer to perform a crossover and inflate an appropriately sized peripheral balloon in the iliac artery. Once the vessel is closed, angiography is performed to confirm vascular integrity.
Comments
Clinical results
The VIV procedure is now routinely performed worldwide in patients considered high-risk for reoperation. Initial results have been promising with the majority patients deriving benefit from this procedure (3-6). In cases of aortic regurgitation, the VIV procedure has been shown to completely eliminate regurgitant flow and in cases of aortic stenosis has been shown to increase the effective orifice area along with a reduction in the transaortic gradient. Most commercially available THVs have been tried but the SAPIEN and Evolut R are the two predominantly used due to lower postoperative gradients compared to other designs (3-6). There is the possibility that the Evolut R provides hemodynamic advantage over other models due to its supra-annular design (5). However, each THV design has certain advantages and may be preferred certain circumstances. For example, the SAPIEN valve provides easier access to the coronary ostia and it can also be used in mitral, tricuspid and pulmonary positions due to its shorter height and ability to be crimped in either direction. On the other hand, the Lotus THV may be completely repositioned and retrieved and hence preferred when there is a risk of coronary obstruction or in stentless valves. Experience in treating degenerated SHVs in the mitral, tricuspid and pulmonary position is increasing. The SAPIEN valve has been used in these cases but cases have also been reported with the Melody valve, especially in paediatric population (16).
Catastrophic complications during a VIV procedure include valve embolization secondary to malposition or coronary obstruction (Figure 9). The initial incidence of malposition was between 1–3% and was attributed to experience of the operating team, improper sizing and certain SHV models, especially those with poor fluoroscopic markers and stentless valves (4,5,15). With increased experience, a wider range of THV sizes, availability of devices that can be repositioned, a thorough approach to sizing and positioning has reduced this risk to less than 1%. Most of the time, malposition can be corrected with transcatheter techniques and open heart surgery is rarely required. Similarly, the incidence of coronary obstruction has been reported to be in the range of 1-3% (3-7). Certain valve designs such as those with leaflets outside the stent and stentless valves tend to carry a higher risk (Figures 10,11). Small SHVs are usually implanted in small aortic roots and these are at higher risk of coronary obstruction, requiring coronary stenting or open heart surgery. When anticipated, it is better to place a guidewire and an undeployed stent in the coronary artery before performing VIV as it can be difficult to access the coronary ostium after the VIV procedure. The stent is then withdrawn into the ostium and deployed partially in the aortic root in order to push the SHV leaflets away from the ostium. If this is not possible or unsuccessful, then open surgery is required. Options during open surgery include removal of the VIV complex and replacement of the valve with another SHV or constructing a coronary artery bypass graft to the obstructed coronary artery.
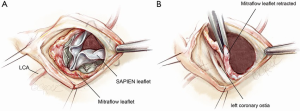
Another important issue to consider is the occurrence of high postoperative residual gradients. This is attributed to the ‘Russian Doll’ effect and is usually observed in SHVs with a true ID less than 21 mm (4). Use of the Evolut R valve is favoured by most users for these SHVs. A technique of fracturing the SHV is being used more and more frequently (17). This essentially involves fracturing of the basal or inflow ring of an SHV by means of a balloon in order to increase the inflow diameter by at least one size. This allows optimal expansion of the THV device and thus reduces gradients. The risks of annular rupture and damage to the THV leaflets with a high-pressure balloon have been cited as two possible concerns with this technique and further evaluation is needed to prove its safety and efficacy. As a solution to this issue in the future, SHV designs with an expandable feature will be become available.
Finally, concerns regarding thrombosis and leaflet immobility have been raised (18). The need for postoperative anticoagulation following the VIV procedure is not currently clear. There have been cases reported of early or delayed leaflet thickening or thrombosis responding to anticoagulation. It is our policy to warfarinize patients, with a target INR of 2.5, for the first three months postoperatively provided there is no contraindication to anticoagulation. In addition, we recommend warfarin and a single antiplatelet agent if some form of coronary stenting was performed during the VIV procedure, due to the possible risk of sudden death in cases where left main stenting was performed to treat perioperative coronary obstruction.
Advantages
The VIV procedure has the following advantages:
- Avoidance of reoperation and its associated risks;
- Shorter operative duration;
- Faster recovery;
- Avoidance of blood transfusions.
As a result, VIV can provide an excellent solution to patients who do not want a mechanical valve but at the same time want a 30-year interval between a further operation (Figure 12).
Caveats
The VIV procedure is a promising new therapy for treating a degenerated SHV. However, long-term data are needed to provide a clearer understanding of the complex issues resulting from interactions between two types of bioprosthesis before it can be recommended as a first-line treatment.
Patient selection
Patient selection is critical to avoid suboptimal results and complications. If coronary obstruction is anticipated, bailout equipment such as CPB and coronary stenting should be readily available. The choice of THV should be determined by the type of SHV and experience of the team. Techniques such as valve fracturing and coronary stenting should only be reserved for those patients for whom the conventional operation is not possible.
Stentless valves
Stentless valves pose a unique challenge (4,5,14). In general, we refer to the sizing information available in the Aortic VIV App but also perform measurements on CT scans. A stentless valve should be treated as a native valve with respect to measurements. The procedure is challenging as there are no fluoroscopic markers and most valves are regurgitant. Hence, techniques such as placement of a pigtail catheter at the base of a leaflet, multiple contrast injections and placement of a wire in the left main coronary artery are critical. Slow deployment and the use of retrievable devices have facilitated this procedure. The risk of coronary obstruction is higher and therefore we always protect the coronary arteries.
Mitral VIV
All SHVs in in the mitral position are stented and their appearance is similar to their counterparts in the aortic position. Greatest experience is with the SAPIEN valve (5). Either a transapical or trans-septal approach can be used. Oversizing is critical as cases of delayed embolization have been reported. Attention must also be given to the possibility left ventricular outflow tract obstruction (LVOTO), the risk of which can be determined by preoperative CT and echocardiography.
Tricuspid VIV
All the SHVs used are stented and, depending on the anatomy, SAPIEN implantation is achieved through a TF venous, transjugular or direct atrial approach. Sizing is similar to that for a mitral VIV (5).
Pulmonic VIV
Either the SAPIEN or Melody valve can be used depending on size. The approach is usually TF venous or transjugular (5).
Acknowledgements
None.
Footnote
Conflicts of Interest: Consultant to Edward Lifesciences, Medtronic Inc., Boston Scientific, Abbott, 4Tech.
References
- Bapat VN. The new "tenth commandment"? EuroIntervention 2016;12:420-1. [Crossref] [PubMed]
- Niclauss L, von Segesser LK, Ferrari E. Aortic biological valve prosthesis in patients younger than 65 years of age: transition to a flexible age limit? Interact Cardiovasc Thorac Surg 2013;16:501-7. [Crossref] [PubMed]
- Bapat V, Attia R, Redwood S, et al. Use of transcatheter heart valves for a valve-in-valve implantation in patients with degenerated aortic bioprosthesis: technical considerations and results. J Thorac Cardiovasc Surg 2012;144:1372-9; discussion 1379-80. [Crossref] [PubMed]
- Dvir D, Webb J, Brecker S, Bleiziffer S, et al. Transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves: results from the global valve-in-valve registry. Circulation 2012;126:2335-44. [Crossref] [PubMed]
- Conradi L, Silaschi M, Seiffert M, et al. Transcatheter valve-in-valve therapy using 6 different devices in 4 anatomic positions: Clinical outcomes and technical considerations. J Thorac Cardiovasc Surg 2015;150:1557-65, 1567.e1-3; discussion 1565-7.
- Mylotte D, Lange R, Martucci G, et al. Transcatheter heart valve implantation for failing surgical bioprostheses: technical considerations and evidence for valve-in-valve procedures. Heart 2013;99:960-7. [Crossref] [PubMed]
- Blanke P, Soon J, Dvir D, et al. Computed tomography assessment for transcatheter aortic valve in valve implantation: The vancouver approach to predict anatomical risk for coronary obstruction and other considerations. J Cardiovasc Comput Tomogr 2016;10:491-9. [Crossref] [PubMed]
- Urena M, Nombela-Franco L, Doyle D, et al. Transcatheter aortic valve implantation for the treatment of surgical valve dysfunction ("valve-in-valve"): assessing the risk of coronary obstruction. J Card Surg 2012;27:682-5. [Crossref] [PubMed]
- Bapat V, Mydin I, Chadalavada S, et al. A guide to fluoroscopic identification and design of bioprosthetic valves: a reference for valve-in-valve procedure. Catheter Cardiovasc Interv 2013;81:853-61. [Crossref] [PubMed]
- Bapat VN, Attia R, Thomas M. Effect of valve design on the stent internal diameter of a bioprosthetic valve: a concept of true internal diameter and its implications for the valve-in-valve procedure. JACC Cardiovasc Interv 2014;7:115-27. [Crossref] [PubMed]
- Bapat V. Valve-in-valve apps: why and how they were developed and how to use them. EuroIntervention. 2014 Sep;10 Suppl U:U44-51.
- Bapat V, Adams B, Attia R, et al. Neo-annulus: a reference plane in a surgical heart valve to facilitate a valve-in-valve procedure. Catheter Cardiovasc Interv 2015;85:685-91. [Crossref] [PubMed]
- Bapat VN, Attia RQ, Condemi F, et al. Fluoroscopic guide to an ideal implant position for Sapien XT and CoreValve during a valve-in-valve procedure. JACC Cardiovasc Interv 2013;6:1186-94. [Crossref] [PubMed]
- Bapat V, Davies W, Attia R, et al. Use of balloon expandable transcatheter valves for valve-in-valve implantation in patients with degenerative stentless aortic bioprostheses: Technical considerations and results. J Thorac Cardiovasc Surg 2014;148:917-22; discussion 922-4. [Crossref] [PubMed]
- Noorani A, Radia R, Bapat V. Challenges in valve-in-valve therapy. J Thorac Dis 2015;7:1501-8. [PubMed]
- Shuto T, Kondo N, Dori Y, et al. Percutaneous transvenous Melody valve-in-ring procedure for mitral valve replacement. J Am Coll Cardiol 2011;58:2475-80. [Crossref] [PubMed]
- Chhatriwalla AK, Allen KB, Saxon JT, et al. Bioprosthetic Valve Fracture Improves the Hemodynamic Results of Valve-in-Valve Transcatheter Aortic Valve Replacement. Circ Cardiovasc Interv 2017.10. [PubMed]
- Vahidkhah K, Javani S, Abbasi M, et al. Blood Stasis on Transcatheter Valve Leaflets and Implications for Valve-in-Valve Leaflet Thrombosis. Ann Thorac Surg 2017;104:751-9. [Crossref] [PubMed]