Secondary tracheal tumors: a systematic review
Introduction
Secondary tracheal tumors are defined as tumors in, but not of the trachea and comprise a wide spectrum of tumor histologies and stages. There are no population-based data for these tumors, and in clinical practice these lesions are probably more common than primary neoplasms. A thoracic surgeon probably encounters tracheal obstruction by metastatic lymph nodes more often than invasion by adjacent primary tumors. Since natural history and prognosis are related to the tumor of origin, their management and prognosis may differ considerably from primary tracheal neoplasms; a correct diagnosis with attribution to the original tumor is therefore important. Tracheal invasion by metastasis may further be categorized as metachronous when the diagnosis is made after a disease-free interval, in contrast to synchronous diagnosis when primary malignancy coincides with tracheal invasion.
Secondary tracheal tumors may arise from hematogenous or lymphatic sites of metastasis, or by direct extension from adjacent structures including thyroid or esophagus. A useful and detailed classification of tracheobronchial metastases was proposed by Kiryu and associates based on the relationship of the primary tumor to the trachea (1). Type 1 tumors are direct metastases to the bronchus; type 2, invasion by an adjacent parenchymal lesion; type 3, invasion by lymph nodes; and type 4, peripheral lesion extending proximally along the airway wall. Most of the studies that we reviewed below reported type 1 tumors from extrapulmonary sites or type 2 tumors from the thyroid. We suspect, however, that invasion by adjacent lymph nodes metastatic from lung, esophageal, head and neck or other cancer is the most frequent form of metastasis; this type would be less represented in reports because the trachea is not the sole focus of attention in progressive disease and the survival of patients after diagnosis is short.
The true incidence of secondary tracheal tumors is unknown. One of the earliest cases of endotracheal metastasis was reported in 1954 in a case of colon cancer metastatic to the trachea (2). Existing epidemiological studies mainly focus on endobronchial metastases and contain different exclusion criteria. In an autopsy study of 1,000 consecutive cases of malignant neoplasms of epithelial origin from 1943 to 1947, six endotracheal metastases originated from primary sites of lung (3), larynx (1) and breast (1); however, this study excluded cases of direct extension to adjacent organs (4). Another study analyzing 1,359 consecutive autopsies from 1968 to 1971 found a 2% incidence of endobronchial metastases and 0.8% of endotracheal metastasis from primary thyroid cancer (1) and melanoma (1); cases of lymphoma, central nervous system tumors and primary lung neoplasms were excluded (3). A literature review from 1966 to 2002 found 204 confirmed cases of endobronchial metastases from extrapulmonary solid tumors, while 4% were located in the trachea (5).
We are unaware of any prior collective analysis of secondary tracheal tumors. In this study, we conduct a systematic review of secondary tracheal tumors based on studies available in the MEDLINE database to analyze the evolution of practice patterns and assess the relative merits of nonoperative, bronchoscopic and surgical therapy. The aim of this study is to provide a guide to multidisciplinary management of patients presenting with airway obstruction from secondary tracheal tumors.
Methods
Literature search strategy
A systematic review was performed. MEDLINE was searched for original published studies using the terms: “tracheal metastasis” OR “secondary tracheal tumors” OR “endotracheal metastasis” OR “tracheobronchial tumors”.
Eligibility criteria
Studies eligible for this systematic review included patients with metastatic disease to the trachea or primary tumors secondarily involving the trachea. We included individual case reports, single institutional case series and multi-institutional case series while excluding reviews, editorials, expert opinions and studies that lacked abstracts. Several reports collected primary and secondary tracheal tumors, or tracheal and bronchial tumors; the data regarding secondary tracheal tumors were specifically extracted and included in this study. For repeated publications reporting similar patients from the same institution, the most recent data set was included [e.g., (6-8)].
Data extraction and critical appraisal
One reviewer (ML Madariaga) reviewed each included study and extracted data from text, tables and figures. Another author reviewed the extracted data (HA Gaissert). All data were entered into a standardized database.
Statistical analysis
Results will be presented as means and ranges where applicable. Kaplan-Meier survival analysis was performed for treatment groups of ≥10 patients derived from individual studies.
Outcome measures
The primary endpoints included symptom relief, overall survival, disease recurrence and 30-day postoperative or post-procedure complications. Secondary endpoints included tumor histology, location of tracheal tumor, and treatment.
Surgical technique
Techniques to resect tracheal invasion were classified as window resection (resection of the anterior tracheal wall only), shave resection (partial thickness resection of the trachea), segmental tracheal resection (circumferential full-thickness resection), exenteration (resection of trachea, esophagus, larynx and other cervicomediastinal structures), and debulking (less than complete resection), the latter by endoscopic or open methods.
Results
Quantity of evidence
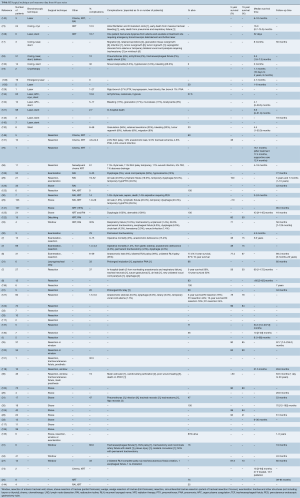
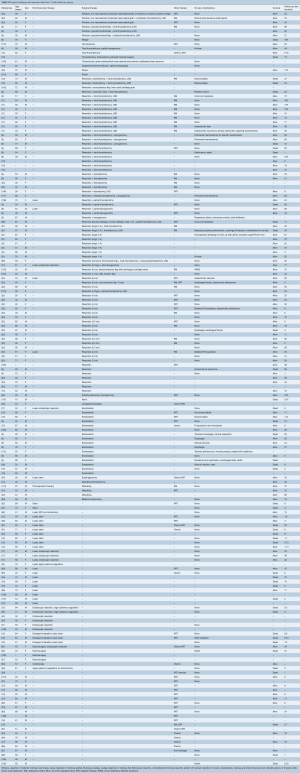
After applying our search criteria, 2,866 studies were identified. Manual evaluation of abstracts and full-length articles identified 160 relevant publications. There were 64 individual case reports and 96 case series (consisting of more than one case). Of the 96 case series, 17 series contained enough details to extract individual patient data; these 110 patients were analyzed with the individual case reports (8-24). Forty-five case series reported exclusively on tracheal invasion by thyroid cancer (Table S1). A total number of 2,242 patients were included in this study, 174 patients in individual case reports and 2,068 patients in case series.
Full table
Quality of evidence
All were retrospective, observational series, and there were no randomized controlled or prospective trials. Thirty retrospective case series included bronchial as well as tracheal lesions, or primary in addition to secondary tracheal tumors, but only provided outcome measures based on the general study population (Table S1). Where possible, data relevant to secondary tracheal tumors were specifically extracted. However, to facilitate interpretation of the general outcome measures in case series, the number of patients with tracheal tumors was compared with the total number of patients in the study (Tables S2,S3). Data unavailable for extraction were left blank in the accompanying tables.
Full table
Full table
Patient demographics
Among 2,242 patients, mean age of patients was 58.5 years and 58.4% were female. The most common histologic types were well-differentiated thyroid cancer (74%), unspecified esophageal cancer (5.93%), and squamous cell carcinoma of the lung (2.77%) (Table 1). In 9.5% of patients, the tumor was not otherwise specified or there was a mix of tumor histology not defined for each patient (Table 1).
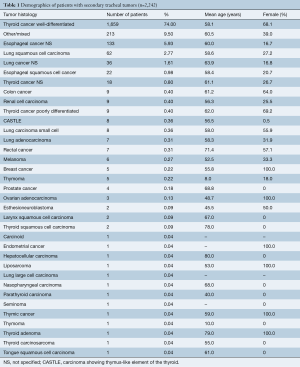
Full table
Symptoms
The presence of symptoms was recorded for 50.4% (n=1,130) of included cases (Table 2). Among 95.2% of patients, the leading symptoms were dyspnea (31.8%), neck mass (19.5%), voice change or hoarseness (15.0%), and hemoptysis (13.7%), while 4.8% were asymptomatic.
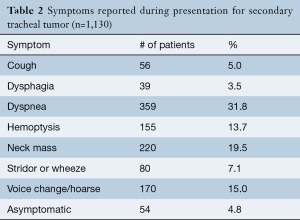
Full table
Concurrent metastases
Among case series of well-differentiated thyroid cancer, lung metastases were present in 13.8% (range, 6.5% to 20.0%) of patients at the time of or prior to diagnosis (7,25-31) and esophageal involvement in 29.0% (range, 4.9% to 62.0%) (6,25,29,31-36). Lung metastases were also present at or prior to secondary tracheal tumor diagnosis in patients with renal cell carcinoma (22%), rectal cancer (85%), melanoma (16.7%), esophageal cancer (3%), colon cancer (33.3%), carcinoma with thymus-like differentiation (CASTLE; 12.5%) and breast cancer (40%) (Table S1). Individual patients with liposarcoma and hepatocellular carcinoma also had lung metastasis (37,38).
Interval between initial diagnosis and secondary tracheal tumor presentation
The time interval between initial tumor diagnosis and secondary tracheal involvement was known in 622 (27.7%) patients, as shown in Table 3. Two thirds of patients presented with delay after diagnosis of the primary tumor. Patients with renal cell carcinoma had the longest median metachronous interval (90.0 months), followed by ovarian (84.0 months) and breast cancer (72.0 months). The shortest median metachronous interval was observed in squamous cell carcinoma of the lung (14.5 months), esthesioneuroblastoma (15.0 months) and adenocarcinoma of the lung (16.5 months). In one study collecting data from patients with various secondary tracheal tumors, the median metachronous interval was 7.3 months (121).
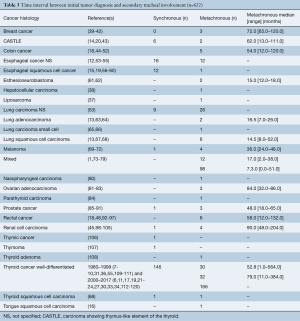
Full table
Assessment of treatment
Data regarding treatment was available for 1,527 patients in case series and 170 patients in individual case reports (detailed in Tables S2,S3). Altogether, 21.7% of patients underwent bronchoscopic management, 32.2% of patients underwent radiation and 76.8% of patients underwent surgery.
In case series, data about treatment and outcome was available in 1,527 patients. The follow-up interval ranged from 12.0 to 97.7 months. Treatment included bronchoscopic management alone in 19%, bronchoscopy and chemotherapy/radiation in 2%, surgical management alone in 50.6%, surgical management and chemotherapy/radiation in 27.3%, and chemotherapy/radiation alone in 0.9%. Surgical therapy consisted of circumferential resection in 41.9%, shave resection in 36.6%, window resection in 11.1%, exenteration in 4.5%, debulking in 1%, and other procedures in 2% (Table S2).
In individual case reports, data regarding treatment and outcome was available in 170 patients. The median follow-up interval was 21 months (range, 0.03–183 months). Treatment was characterized as bronchoscopic alone in 16.5%, bronchoscopy with chemotherapy/radiation in 6.5%, surgical management alone in 32.9%, surgical management with chemotherapy/radiation in 28.3%, combined surgical and bronchoscopic management in 2.4%, chemotherapy/radiation alone in 11% and in 2.4%, a combination of resection, bronchoscopy and chemotherapy/radiation. Surgical therapy for tracheal invasion included circumferential resection in 65%, exenteration in 14.2%, window resection in 5.4%, shave resection in 2.7%, and debulking in 2.7%. The average length of resected circumferential trachea measured 3.7 rings or 3.8 cm (Table S3).
We are not aware of any combined resections of trachea and the entire esophagus with successful reconstruction of tracheal continuity. In the literature of invasive thyroid cancer, there was one case of laryngotracheoesophagectomy with reconstruction of the digestive tract with a forearm free flap (109). There were 28 cases of circumferential tracheal and partial-thickness or partial-circumference esophageal resection with reconstruction (6,8,10,25,29) and in addition two cases of combined partial resection of trachea and esophagus (25). One series reported concomitant unspecified esophageal resection with tracheal shave (n=5), tracheal window resection (n=2) and circumferential tracheal resection with reconstruction (n=1) (31). Two series of aerodigestive tract invasion by thyroid cancer reported patients who underwent partial to circumferential tracheal and esophageal resections, but did not specify how many patients had concomitant resections (33,36). There was one patient with esophageal cancer who underwent tangential tracheal resection, spiral tracheoplasty and esophagectomy (19) and two patients with esophageal cancer who underwent partial esophagectomy with partial tracheal resection and muscle flap reconstruction (15).
Six case series of thyroid cancer invading the trachea directly analyzed survival associated with different treatment options. One study from China with 156 patients with thyroid cancer showed that 5-year survival was 100% with 0 cancer recurrence after circumferential resection compared to 5-year survival of <10% with 54% cancer recurrence after tracheal shaving (29); circumferential tracheal resection showed improved survival over tracheal shaving in other studies as well (31). However, other studies did not demonstrate significant difference in survival outcomes between tracheal shave and circumferential resection (122,123). In one study from Japan containing 114 patients, those undergoing shave resection had better 5-year survival (99%) than those undergoing circumferential resection (71%) (124); this result was also seen in a study of 65 patients from Korea (35); there was no analysis of survival beyond 5 years in these slow-growing tumors.
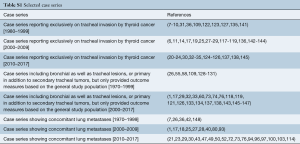
Assessment of complications
Data regarding complications after treatment were available in 54.4% of patients from case series and are detailed in Table 4 and Tables S2,S3. The rate of complications ranged from 12.5% to 16.7% for bronchoscopic management with chemotherapy/radiation, 0.0% to 58.0% for bronchoscopy alone, 0.0% to 78.0% for resection alone and 0.0% to 61.0% for surgery with chemotherapy/radiation.
The most common complications after bronchoscopic intervention was bleeding (range, 17–33%), formation of granulation tissue after stent insertion (range, 17–58%), retained sputum (range, 11–33%) and stent migration (8%) (Table 4). Death during hospitalization occurred in 0.01% to 0.04%.
Following resection, the most common complications were anastomotic leak (range, 0.04–23.8%), chyle leak (range, 4.3–22%), new recurrent laryngeal nerve paralysis (range, 0.04–55%), esophageal fistula (5–6%), unplanned permanent tracheostomy (range, 4.3–50%), respiratory failure or prolonged intubation (10%), tracheal stenosis (5%) and wound infection (range, 1.4–11%). In one study of thyroidectomy, lymph node dissection and shave resection of the trachea with radioactive iodine and selected adjuvant external beam radiation, all patients experienced dysphagia after radiation (125). Hospital mortality ranged from 0.02% to 1.4% in this group.
Data on complications were available for 70% of individual case reports (121 of 174 patients). There were no complications in 70% of all cases with available data. The most frequent complications were anastomotic leak in 9.0%, tracheal stenosis in 3.3% and unplanned permanent tracheostomy in 3.3%. Additional complications included respiratory failure or prolonged intubation in 2.5%, new recurrent laryngeal nerve permanent paralysis in 2.5%, esophageal leak or fistula in 2.4%, dysphagia in 2.5% and hospital death in 2.5%.
Assessment of survival
In our review of case series, 95% of patients had data regarding survival outcomes as shown in Table S2. Following surgical therapy, 5-year survival ranged from <10% to 100% and 10-year survival ranged from 15% to 90%. Reported median survival after bronchoscopic management ranged from 0.3 to 75.0 months, whereas median survival in the surgery treatment group ranged from 3 to 207 months. In the case series, median survival in patients with non-thyroid malignancy ranged from 1 to 18 months and in thyroid malignancy from 8.0 to 112.8 months. Median 5-year survival in patients with thyroid malignancy was 74.2% [range, <10% after shave resection (29) to 100%].
The 174 patients in individual case reports were grouped by treatment modality, cancer histology and available survival outcomes data to conduct Kaplan-Meier survival analysis (Table S3). Overall 5-year survival was 80% after resection alone (n=47), 75% after resection with chemotherapy/radiation (n=44), 25% after bronchoscopic intervention with or without stent placement (n=19) and less than 20% after bronchoscopy with chemotherapy/radiation (n=10). Overall 5-year survival was 42% in patients with non-thyroid and 78% in thyroid malignancy; overall 10-year survival was 42% in patients with non-thyroid and 55% in thyroid cancer.
Assessment of recurrence
Data on recurrence were extracted from 13 case series identified for collective analysis (7,17,27,29,33,35,73,124,126,135-138) and nine individual case reports (14,15,17,44,56,61,81,98,139) and are listed in Table S4. The disease-free interval ranged from 1 to 58 months. Among patients with thyroid cancer who underwent bronchoscopic intervention, the incidence of recurrence ranged from 17% to 82%. Recurrence after segmental tracheal resection for thyroid cancer ranged from 0% to 34% and after tracheal shave resection from 4.7% to 54%. One study comparing shave and segmental tracheal resection for thyroid cancer found the local recurrence higher after shave resection [54% versus 0%, (29)]. Data on recurrence in patients with other cancer histology were too few to draw meaningful conclusions.
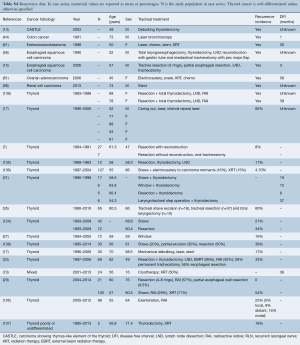
Full table
Discussion
Few individual centers amass a meaningful clinical experience in the management of tracheal tumors, and far fewer yet in secondary tracheal malignancy. The extraction of information from data useful to the clinical surgeon is therefore difficult and requires critical reading between the lines. Case reports often emphasize perceived successes, while palliative intervention in patients with advanced, progressive malignant disease is probably underreported. There are, however, several principal findings and conclusions we consider central to the understanding of secondary tracheal malignancy.
- The distinction between invasion by a primary tumor of a structure adjacent to the trachea and a lymphatic or hematogenous metastasis is important for treatment and the assessment of prognosis;
- Obtaining the histologic diagnosis is valuable even after the distinction outlined by point I) is made, since more than one tumor type may arise from organs or lymph nodes adjacent to the trachea;
- Even when considering the guarded prognosis of most unreported secondary tracheal malignancies, segmental tracheal resection is successful in patients who are carefully selected for favorable histology and limited airway involvement;
- While secondary tracheal tumors often require the coordinated deployment of multiple modalities, a tracheal surgeon experienced in segmental resection is a key member of this group;
- Oncologically doubtful interventions on the trachea, specifically shave and window resections, have no proven value in treatment with curative intent. Neither windows nor shaves permit the reliable assessment of margins and persist in use only because the consequence of incomplete resection is not discovered for many months or years. Since the tumors often occur in younger patients, observation of survival should extend to 20 or even 30 years to capture meaningful adverse events;
- Cervical exenteration involves resection of both trachea and esophagus, but does not result in end-to-end tracheal reconstruction. An important consequence of esophageal resection is the loss of tracheal blood supply as the vascular arcades on either side are disrupted. Even though any resection of the esophagus above the carina inevitably renders the trachea ischemic, no sequelae occur in absence of a tracheal anastomosis. The addition of a tracheal anastomosis to complete esophageal resection poses a high risk of failure even with only modest tension due to ischemia.
The major limitation of this study is its reliance on case studies or case series that have incomplete or heterogeneous information. Most studies examined patients with endobronchial metastases, where cases of endotracheal metastases are few; other studies presented outcomes based on mixed tumor histology of secondary tracheal tumors. We mitigated this bias by extracting outcomes values pertinent to secondary tracheal tumors where possible. Comparison of survival outcomes after different treatment modalities is rare because tumor histology and stage are not controlled. A more homogeneous data set would allow us to assess the relative utility of therapeutic interventions and their specific impact on morbidity and mortality. The interpretation of complications is challenging in a collective review as patient selection and operative standards vary widely among institutions. Complications were reported in half of all case reports and may have inspired publication. Similar limitations were noted by a review of laryngotracheal invasion by thyroid carcinoma from 1971 to 1990 that included 595 patients from 20 studies with airway invasion (140) and a review of endobronchial metastases from extrapulmonary solid tumors from 1962 to 2002 (5).
In conclusion, this comprehensive systematic review of secondary tracheal tumors highlights common presentations, modes of treatment and general outcomes. Patients with secondary tracheal tumors should be assessed for overall prognosis and impediments to quality of life. An individualized treatment approach involving surgical, bronchoscopic and medical therapies can then be utilized to optimize clinical outcomes.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kiryu T, Hoshi H, Matsui E, et al. Endotracheal/endobronchial metastases: Clinicopathologic study with special reference to developmental modes. Chest 2001;119:768-75. [Crossref] [PubMed]
- Divertie MB, Schmidt HW. Tracheal obstruction from metastatic carcinoma of the colon: Report of case. Proc Staff Meet Mayo Clin 1954;29:403-5. [PubMed]
- Braman SS, Whitcomb ME. Endobronchial metastasis. Arch Intern Med 1975;135:543-7. [Crossref] [PubMed]
- Abrams HL, Spiro R, Goldstein N. Metastases in carcinoma; analysis of 1000 autopsied cases. Cancer 1950;3:74-85. [Crossref] [PubMed]
- Sørensen JB. Endobronchial metastases from extrapulmonary solid tumors. Acta Oncol 2004;43:73-9. [Crossref] [PubMed]
- Gaissert HA, Honings J, Grillo HC, et al. Segmental laryngotracheal and tracheal resection for invasive thyroid carcinoma. Ann Thorac Surg 2007;83:1952-9. [Crossref] [PubMed]
- Grillo HC, Suen HC, Mathisen DJ, et al. Resectional management of thyroid carcinoma invading the airway. Ann Thorac Surg 1992;54:3-9; discussion 9-10. [Crossref] [PubMed]
- Grillo HC, Zannini P. Resectional management of airway invasion by thyroid carcinoma. Ann Thorac Surg 1986;42:287-98. [Crossref] [PubMed]
- Shvili Y, Zohar Y, Buller N, et al. Conservative surgical management of invasive differentiated thyroid cancer. J Laryngol Otol 1985;99:1255-60. [Crossref] [PubMed]
- Talpos GB. Tracheal and laryngeal resections for differentiated thyroid cancer. Am Surg 1999;65:754-9; discussion 759-60. [PubMed]
- Yang CC, Lee CH, Wang LS, et al. Resectional treatment for thyroid cancer with tracheal invasion: A long-term follow-up study. Arch Surg 2000;135:704-7. [Crossref] [PubMed]
- Chan KP, Eng P, Hsu AA, et al. Rigid bronchoscopy and stenting for esophageal cancer causing airway obstruction. Chest 2002;122:1069-72. [Crossref] [PubMed]
- Chong S, Kim TS, Han J. Tracheal metastasis of lung cancer: Ct findings in six patients. AJR Am J Roentgenol 2006;186:220-4. [Crossref] [PubMed]
- Chow SM, Chan JK, Tse LL, et al. Carcinoma showing thymus-like element (castle) of thyroid: Combined modality treatment in 3 patients with locally advanced disease. Eur J Surg Oncol 2007;33:83-5. [Crossref] [PubMed]
- Doki Y, Yasuda T, Miyata H, et al. Salvage lymphadenectomy of the right recurrent nerve node with tracheal involvement after definitive chemoradiation therapy for esophageal squamous cell carcinoma: Report of two cases. Surg Today 2007;37:590-5. [Crossref] [PubMed]
- Nguyen BD, Ram PC, Roarke MC. Endotracheal metastasis from squamous cell cancer of the head and neck: Pet/ct imaging. Clin Nucl Med 2008;33:340-1. [Crossref] [PubMed]
- Tsutsui H, Usuda J, Kubota M, et al. Endoscopic tumor ablation for laryngotracheal intraluminal invasion secondary to advanced thyroid cancer. Acta Otolaryngol 2008;128:799-807. [Crossref] [PubMed]
- Galbis Caravajal JM, Sales Badia JG, Trescoli Serrano C, et al. Endotracheal metastases from colon adenocarcinoma. Clin Transl Oncol 2008;10:676-8. [Crossref] [PubMed]
- Wu MH. Spiral tracheoplasty after tangential resection of trachea. Ann Thorac Surg 2009;88:2042-3. [Crossref] [PubMed]
- Tsutsui H, Hoshi M, Kubota M, et al. Management of thyroid carcinoma showing thymus-like differentiation (castle) invading the trachea. Surg Today 2013;43:1261-8. [Crossref] [PubMed]
- Mossetti C, Palestini N, Bruna MC, et al. Segmental tracheal resection for invasive differentiated thyroid carcinoma. Our experience in eight cases. Langenbecks Arch Surg 2013;398:1075-82. [Crossref] [PubMed]
- Lin S, Huang H, Liu X, et al. Treatments for complications of tracheal sleeve resection for papillary thyroid carcinoma with tracheal invasion. Eur J Surg Oncol 2014;40:176-81. [Crossref] [PubMed]
- Dowthwaite S, Friel M, Coman S. Tracheal reconstruction using composite nasal septal graft in patients with invasive thyroid carcinoma. J Laryngol Otol 2015;129 Suppl 1:S16-20. [Crossref] [PubMed]
- Gozen ED, Yener M, Erdur ZB, et al. End-to-end anastomosis in the management of laryngotracheal defects. J Laryngol Otol 2017;131:447-54. [Crossref] [PubMed]
- Nakao K, Kurozumi K, Fukushima S, et al. Merits and demerits of operative procedure to the trachea in patients with differentiated thyroid cancer. World J Surg 2001;25:723-7. [Crossref] [PubMed]
- Wu MH, Tseng YL, Lin MY, et al. Surgical results of 40 patients with malignant tracheobronchial lesions. Respirology 1997;2:255-9. [Crossref] [PubMed]
- Tsukahara K, Sugitani I, Kawabata K. Tracheal resection with end-to-end anastomosis preserving paries membranaceus trachea for patients with papillary thyroid carcinoma. Acta Otolaryngol 2009;129:575-9. [Crossref] [PubMed]
- Tsukahara K, Sugitani I, Kawabata K. Surgical management of tracheal shaving for papillary thyroid carcinoma with tracheal invasion. Acta Otolaryngol 2009;129:1498-502. [Crossref] [PubMed]
- Chen W, Zou S, Wang L, et al. Anastomosis in the absence of a suprahyoid release following circumferential sleeve resection is feasible in differentiated thyroid carcinoma patients with tracheal invasion. Oncol Lett 2017;14:2822-30. [Crossref] [PubMed]
- Shenoy AM, Burrah R, Rao V, et al. Tracheal resection for thyroid cancer. J Laryngol Otol 2012;126:594-7. [Crossref] [PubMed]
- Musholt TJ, Musholt PB, Behrend M, et al. Invasive differentiated thyroid carcinoma: tracheal resection and reconstruction procedures in the hands of the endocrine surgeon. Surgery 1999;126:1078-87; discussion 1087-8. [Crossref] [PubMed]
- Sugitani I, Hasegawa Y, Sugasawa M, et al. Super-radical surgery for anaplastic thyroid carcinoma: A large cohort study using the anaplastic thyroid carcinoma research consortium of japan database. Head Neck 2014;36:328-33. [Crossref] [PubMed]
- Su SY, Milas ZL, Bhatt N, et al. Well-differentiated thyroid cancer with aerodigestive tract invasion: Long-term control and functional outcomes. Head Neck 2016;38:72-8. [Crossref] [PubMed]
- Ebihara M, Kishimoto S, Hayashi R, et al. Window resection of the trachea and secondary reconstruction for invasion by differentiated thyroid carcinoma. Auris Nasus Larynx 2011;38:271-5. [Crossref] [PubMed]
- Kim H, Jung HJ, Lee SY, et al. Prognostic factors of locally invasive well-differentiated thyroid carcinoma involving the trachea. Eur Arch Otorhinolaryngol 2016;273:1919-26. [Crossref] [PubMed]
- Ballantyne AJ. Resections of the upper aerodigestive tract for locally invasive thyroid cancer. Am J Surg 1994;168:636-9. [Crossref] [PubMed]
- Nair S, Kumar P, Ladas G. Intratracheal metastasis secondary to soft tissue liposarcoma. Singapore Med J 2007;48:e81-3. [PubMed]
- Hamai Y, Hihara J, Aoki Y, et al. Airway stenting for tracheal obstruction due to lymph node metastasis of hepatocellular carcinoma. Anticancer Res 2013;33:1761-4. [PubMed]
- Charalabopoulos K, Dalavaga Y, Stefanou D, et al. Direct endobronchial metastasis is a rare metastatic pattern in breast cancer. Int J Clin Pract 2004;58:641-4. [Crossref] [PubMed]
- Koizumi T, Kobayashi N, Kanda S, et al. Diffuse endobronchial wall spread of metastatic breast cancer. Case Rep Oncol 2009;2:77-83. [Crossref] [PubMed]
- Rotolo N, Dominioni L, De Monte L, et al. Metastasis at a tracheostomy site as the presenting sign of late recurrent breast cancer. Head Neck 2013;35:E359-62. [Crossref] [PubMed]
- Garces M, Tsai E, Marsan RE. Endotracheal metastasis. Chest 1974;65:350-1. [Crossref] [PubMed]
- Yano T, Asano M, Tanaka S, et al. Prospective study comparing the new sclerotherapy and hemorrhoidectomy in terms of therapeutic outcomes at 4 years after the treatment. Surg Today 2014;44:449-53. [Crossref] [PubMed]
- Conti JA, Kemeny N, Klimstra D, et al. Colon carcinoma metastatic to the trachea. Report of a case and a review of the literature. Am J Clin Oncol 1994;17:227-9. [Crossref] [PubMed]
- Sakumoto N, Inafuku S, Shimoji H, et al. Endobronchial metastasis from renal cell carcinoma: Report of a case. Surg Today 2000;30:744-6. [Crossref] [PubMed]
- Lee M, Lee YK, Jeon TJ, et al. A case of tracheal metastasis in colon cancer: Detection with 18f-fdg pet/ct. Clin Nucl Med 2015;40:91-2. [Crossref] [PubMed]
- Chun KA. Case reports on the differentiation of malignant and benign intratracheal lesions by 18f-fdg pet/ct. Medicine (Baltimore) 2015;94:e1704. [Crossref] [PubMed]
- Carlin BW, Harrell JH 2nd, Olson LK, et al. Endobronchial metastases due to colorectal carcinoma. Chest 1989;96:1110-4. [Crossref] [PubMed]
- Blanc CD, Donati G, Carbone E, et al. Tracheal metastasis. J Craniofac Surg 2015;26:982-3. [Crossref] [PubMed]
- Tabacchi E, Ghedini P, Cambioli S, et al. Endotracheal metastasis from colorectal cancer. Eur J Nucl Med Mol Imaging 2015;42:1335-6. [Crossref] [PubMed]
- Coriat R, Diaz O, de la Fouchardiere C, et al. Endobronchial metastases from colorectal adenocarcinomas: Clinical and endoscopic characteristics and patient prognosis. Oncology 2007;73:395-400. [Crossref] [PubMed]
- Nakamura T, Tajima T, Ogimi T, et al. Expandable metallic stent for endobronchial metastasis from colorectal cancer: Reports of 2 cases. Tokai J Exp Clin Med 2017;42:79-84. [PubMed]
- Song JU, Park HY, Kim H, et al. Prognostic factors for bronchoscopic intervention in advanced lung or esophageal cancer patients with malignant airway obstruction. Ann Thorac Med 2013;8:86-92. [Crossref] [PubMed]
- Miwa K, Matsuo T, Takamori S, et al. Temporary stenting for malignant tracheal stenosis due to esophageal cancer: A case report. Jpn J Clin Oncol 2002;32:27-9. [Crossref] [PubMed]
- Grillo HC. Tracheal tumors: Surgical management. Ann Thorac Surg 1978;26:112-25. [Crossref] [PubMed]
- Kimura A, Nimura Y, Hayakawa N, et al. A new method of anterior mediastinal tracheostomy following resection of cervical esophagus and the larynx: Report of a case. Surg Today 1994;24:548-51. [Crossref] [PubMed]
- Nicholson DA. Tracheal and oesophageal stenting for carcinoma of the upper oesophagus invading the tracheo-bronchial tree. Clin Radiol 1998;53:760-3. [Crossref] [PubMed]
- Van Raemdonck D, Van Cutsem E, Menten J, et al. Induction therapy for clinical t4 oesophageal carcinoma; a plea for continued surgical exploration. Eur J Cardiothorac Surg 1997;11:828-37. [Crossref] [PubMed]
- Bartolo K, Fsadni P. Stridor: a rare presentation of oesophageal malignancy. BMJ Case Rep 2015;2015. doi: 10.1136/bcr-2015-212408. [Crossref]
- Garrido T, Maluf-Filho F, Sallum RA, et al. Endobronchial ultrasound application for diagnosis of tracheobronchial tree invasion by esophageal cancer. Clinics (Sao Paulo) 2009;64:499-504. [Crossref] [PubMed]
- Mattavelli F, Pizzi N, Pennacchioli E, et al. Esthesioneuroblastoma metastatic to the trachea. Acta Otorhinolaryngol Ital 2009;29:164-8. [PubMed]
- Franklin D, Miller RH, Bloom MG, et al. Esthesioneuroblastoma metastatic to the trachea. Head Neck Surg 1987;10:102-6. [Crossref] [PubMed]
- Kanzaki M, Onuki T, Tatebayashi T, et al. Bilateral endobronchial metastasis in postoperative stage i pulmonary adenocarcinoma. Diagn Ther Endosc 2000;6:141-5. [Crossref] [PubMed]
- Zhang Z, Mao Y, Chen H, et al. Endotracheal and endobronchial metastases in a patient with stage i lung adenocarcinoma. Ann Thorac Surg 2014;97:e135-7. [Crossref] [PubMed]
- De S. Tracheal metastasis of small cell lung cancer. Lung India 2009;26:162-4. [Crossref] [PubMed]
- Liebling M, Boyd M, Rubio E, et al. Airway metastasis of small cell lung carcinoma: A rare presentation. Thorac Cancer 2013;4:461-4. [Crossref] [PubMed]
- Youn HC, Kim YH, Lee YK, et al. Multiple endotracheal and endobronchial metastases after pneumonectomy for a primary lung cancer: A case report. Thorac Cancer 2013;4:453-6. [Crossref] [PubMed]
- Ko YS, Hwang TS, Han HS, et al. Primary pure squamous cell carcinoma of the thyroid: Report and histogenic consideration of a case involving a braf mutation. Pathol Int 2012;62:43-8. [Crossref] [PubMed]
- Capaccio P, Peri A, Fociani P, et al. Flexible argon plasma coagulation treatment of obstructive tracheal metastatic melanoma. Am J Otolaryngol 2002;23:253-5. [Crossref] [PubMed]
- Shelton T, Cambron S, Seltzer M, et al. Tracheal metastasis from melanoma detected with 18f-fdg pet/ct. Clin Nucl Med 2013;38:815-7. [Crossref] [PubMed]
- Purcell P, Meyer T, Allen C. Tracheal mass. Malignant melanoma metastatic to the trachea. JAMA Otolaryngol Head Neck Surg 2015;141:291-2. [Crossref] [PubMed]
- Heyman BM, Chung MM, Lark AL, et al. Endobronchial metastasis from primary anorectal melanoma. Am J Case Rep 2013;14:253-7. [Crossref] [PubMed]
- Ma Q, Shi B, Tian Y, et al. Fibrobronchoscopic cryosurgery for secondary malignant tumors of the trachea and main bronchi. Thorac Cancer 2016;7:459-66. [Crossref] [PubMed]
- Akoglu S, Ucan ES, Celik G, et al. Endobronchial metastases from extrathoracic malignancies. Clin Exp Metastasis 2005;22:587-91. [Crossref] [PubMed]
- Marchioni A, Lasagni A, Busca A, et al. Endobronchial metastasis: An epidemiologic and clinicopathologic study of 174 consecutive cases. Lung Cancer 2014;84:222-8. [Crossref] [PubMed]
- Lee SH, Jung JY, Kim DH, et al. Endobronchial metastases from extrathoracic malignancy. Yonsei Med J 2013;54:403-9. [Crossref] [PubMed]
- Kim JH, Min D, Song SH, et al. Endobronchial metastases from extrathoracic malignancies: Recent 10 years' experience in a single university hospital. Tuberc Respir Dis (Seoul) 2013;74:169-76. [Crossref] [PubMed]
- Salud A, Porcel JM, Rovirosa A, et al. Endobronchial metastatic disease: Analysis of 32 cases. J Surg Oncol 1996;62:249-52. [Crossref] [PubMed]
- Dursun AB, Demirag F, Bayiz H, et al. Endobronchial metastases: A clinicopathological analysis. Respirology 2005;10:510-4. [Crossref] [PubMed]
- Lu H, Chen J, Xie Y, et al. Intrathoracic endotracheal metastasis from nasopharyngeal carcinoma: A first case report and review of the literature. Case Rep Oncol 2010;3:160-4. [Crossref] [PubMed]
- Choi HS, Kim SY, Choi CW, et al. Use of bronchoscopic electrocautery in removing an endotracheal metastasis. Lung Cancer 2007;58:286-90. [Crossref] [PubMed]
- Westerman DE, Urbanetti JS, Rudders RA, et al. Metastatic endotracheal tumor from ovarian carcinoma. Chest 1980;77:798-800. [Crossref] [PubMed]
- Petru E, Friedrich G, Pickel H, et al. Life-threatening tracheal metastasis complicating ovarian cancer--a case report. Gynecol Oncol 1999;74:141-2. [Crossref] [PubMed]
- Wynne AG, van Heerden J, Carney JA, et al. Parathyroid carcinoma: Clinical and pathologic features in 43 patients. Medicine (Baltimore) 1992;71:197-205. [Crossref] [PubMed]
- Cetinkaya E, Ozgul MA, Cam E, et al. Endobronchial metastasis from transitional cell carcinoma of the urinary bladder. J Bronchology Interv Pulmonol 2011;18:158-60. [Crossref] [PubMed]
- Gerogianni I, Gravas S, Papadopoulos D, et al. Endobronchial metastasis from prostate cancer. Int Urol Nephrol 2008;40:961-4. [Crossref] [PubMed]
- Taylor H, Braude S. Lobar collapse due to endobronchial metastatic prostatic carcinoma: Re-expansion with antiandrogen treatment. Thorax 1990;45:66-7. [Crossref] [PubMed]
- Lee DW, Ro JY, Sahin AA, et al. Mucinous adenocarcinoma of the prostate with endobronchial metastasis. Am J Clin Pathol 1990;94:641-5. [Crossref] [PubMed]
- Scoggins WG, Witten JA Jr, Texter JH Jr, et al. Endobronchial metastasis from prostatic cancer in patient with renal cell carcinoma. Urology 1978;12:207-9. [Crossref] [PubMed]
- Kenny JN, Smith WL, Brawer MK. Endobronchial metastases from prostatic carcinoma. Ann Thorac Surg 1988;45:223-4. [Crossref] [PubMed]
- Lalli C, Gogia H, Raju L. Multiple endobronchial metastases from carcinoma of prostate. Urology 1983;21:164-5. [Crossref] [PubMed]
- Watanabe S, Oda M, Ohta Y, et al. Endotracheal metastasis of rectal cancer. Eur J Cardiothorac Surg 2002;21:924. [Crossref] [PubMed]
- Piazza C, Bolzoni A, Peretti G, et al. Thyroid metastasis from rectal adenocarcinoma involving the airway treated by crico-tracheal resection and anastomosis: The role of palliative surgery. Eur Arch Otorhinolaryngol 2004;261:469-72. [Crossref] [PubMed]
- Shim HK, Kwon HW, Kim TS, et al. Endotracheal metastasis seen on fdg pet/ct in a patient with previous colorectal cancer. Nucl Med Mol Imaging 2010;44:294-6. [Crossref] [PubMed]
- Serbanescu GL, Anghel RM. Can endobronchial or endotracheal metastases appear from rectal adenocarcinoma? J Med Life 2017;10:66-9. [PubMed]
- Choi IY, Lee KY, Lee JH, et al. Tracheal metastasis from rectal cancer: A case report and review of the literature. Balkan Med J 2013;30:120-2. [Crossref] [PubMed]
- Rosado Dawid NZ, Villegas Fernandez FR, Rodriguez Cruz Mdel M, et al. Endobronchial metastases of colorectal cancer. Rev Esp Enferm Dig 2016;108:232-3. [PubMed]
- Watanabe H, Uruma T, Tsunoda T, et al. Palliation of malignant tracheal stenosis with a second implantation of an expandable metallic stent under endotracheal intubation. Tokai J Exp Clin Med 2013;38:46-51. [PubMed]
- Shimoyama T, Kojima K, Akamatsu H. Endobronchial metastasis from renal cell carcinoma resected 9 years before; report of a case. Kyobu Geka 2008;61:415-8. [PubMed]
- Parghane RV, Sood A, Vaiphei K, et al. Presentation of unusual tracheal metastasis on fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography after 9 years in postnephrectomy patient of renal cell carcinoma: A case report and review of literature. World J Nucl Med 2017;16:240-2. [Crossref] [PubMed]
- Suyama H, Igishi T, Makino H, et al. Bronchial artery embolization before interventional bronchoscopy to avoid uncontrollable bleeding: A case report of endobronchial metastasis of renal cell carcinoma. Intern Med 2011;50:135-9. [Crossref] [PubMed]
- Ciorra AA, Sciacca V, Pistillucci G, et al. Unusual endotracheal and breast metastasis from renal clear cell carcinoma: A case report. Clin Ter 2013;164:e515-7. [PubMed]
- Byard RW. Endobronchial/tracheal metastasis and sudden death. J Forensic Sci 2014;59:1139-41. [Crossref] [PubMed]
- MacMahon H, O'Connell DJ, Cimochowski GE. Pedunculated endotracheal metastasis. AJR Am J Roentgenol 1978;131:713-4. [Crossref] [PubMed]
- Subramanyam NS, Fendley H, Freeman WH. Coughing up of metastatic tumor as the initial clinical manifestation of renal cell carcinoma. J Ark Med Soc 1991;88:86-7. [PubMed]
- Nomori H, Morinaga S, Kobayashi R, et al. Cervical thymic cancer infiltrating the trachea and thyroid. Eur J Cardiothorac Surg 1994;8:222-4. [Crossref] [PubMed]
- Suseelan AV, Ikerionwu SE, Ojukwu JO. Invasive thymoma (a case report). Neoplasma 1979;26:493-7. [PubMed]
- Love RL, Ahsan F, Allison R, et al. Multinodular goitre arising in the tracheal lumen: Implantation or ectopic? J Laryngol Otol 2012;126:100-2. [Crossref] [PubMed]
- Fujimoto Y, Obara T, Ito Y, et al. Aggressive surgical approach for locally invasive papillary carcinoma of the thyroid in patients over forty-five years of age. Surgery 1986;100:1098-107. [PubMed]
- Donnelly MJ, Considine N, McShane DP. Upper airway invasion by well-differentiated thyroid carcinoma. J Laryngol Otol 1993;107:752-4. [Crossref] [PubMed]
- Sadek SA, Dogra TS, Khan MK, et al. Plasmacytoma of the nasopharynx (a case report with a follow-up of twelve years). J Laryngol Otol 1985;99:1289-92. [Crossref] [PubMed]
- Ashford BG, Clark JR. Cricotracheal reconstruction following external beam radiotherapy for recurrent thyroid cancer. ANZ J Surg 2009;79:271-4. [Crossref] [PubMed]
- Barber P, Deiraniya AK, Allen E. Photodynamic therapy for tracheal thyroid metastasis. Photodiagnosis Photodyn Ther 2004;1:99-102. [Crossref] [PubMed]
- Ozkan E, Araz M, Soydal C, et al. Detection of intraluminal tracheal metastasis of thyroid papillary carcinoma by 18f-fdg pet/ct. Clin Nucl Med 2012;37:e160-1. [Crossref] [PubMed]
- Shimizu J, Arano Y, Yachi T, et al. A 90-year-old woman with trachea-invading thyroid cancer requiring four-ring resection of cervical trachea because of airway stenosis. Ann Thorac Cardiovasc Surg 2007;13:341-4. [PubMed]
- Chattopadhyay B, Bhattacharya B, Chatterjee A, et al. Whistle from afar: a case of endotracheal metastasis in papillary thyroid cancer. Case Rep Oncol Med 2012;2012:235062.
- Tomoda C, Uruno T, Takamura Y, et al. Ultrasonography as a method of screening for tracheal invasion by papillary thyroid cancer. Surg Today 2005;35:819-22. [Crossref] [PubMed]
- Martins AS, Melo GM, Valerio JB, et al. Treatment of locally aggressive well-differentiated thyroid cancer. Int Surg 2001;86:213-9. [PubMed]
- Kim KH, Sung MW, Chang KH, et al. Therapeutic dilemmas in the management of thyroid cancer with laryngotracheal involvement. Otolaryngol Head Neck Surg 2000;122:763-7. [Crossref] [PubMed]
- Shigemitsu K, Naomoto Y, Haisa M, et al. A case of thyroid cancer involving the trachea: Treatment by partial tracheal resection and repair with a latissimus dorsi musculocutaneous flap. Jpn J Clin Oncol 2000;30:235-8. [Crossref] [PubMed]
- Okiror L, Jiang L, Oswald N, et al. Bronchoscopic management of patients with symptomatic airway stenosis and prognostic factors for survival. Ann Thorac Surg 2015;99:1725-30. [Crossref] [PubMed]
- Czaja JM, McCaffrey TV. The surgical management of laryngotracheal invasion by well-differentiated papillary thyroid carcinoma. Arch Otolaryngol Head Neck Surg 1997;123:484-90. [Crossref] [PubMed]
- Mellière DJ, Ben Yahia NE, Becquemin JP, et al. Thyroid carcinoma with tracheal or esophageal involvement: Limited or maximal surgery? Surgery 1993;113:166-72. [PubMed]
- Hotomi M, Sugitani I, Toda K, et al. A novel definition of extrathyroidal invasion for patients with papillary thyroid carcinoma for predicting prognosis. World J Surg 2012;36:1231-40. [Crossref] [PubMed]
- Kim YS, Choi JH, Kim KS, et al. The role of adjuvant external beam radiation therapy for papillary thyroid carcinoma invading the trachea. Radiat Oncol J 2017;35:112-20. [Crossref] [PubMed]
- Kim JW, Roh JL, Gong G, et al. Treatment outcomes and risk factors for recurrence after definitive surgery of locally invasive well-differentiated papillary thyroid carcinoma. Thyroid 2016;26:262-70. [Crossref] [PubMed]
- Ishihara T, Kobayashi K, Kikuchi K, et al. Surgical treatment of advanced thyroid carcinoma invading the trachea. J Thorac Cardiovasc Surg 1991;102:717-20. [PubMed]
- George PJ, Garrett CP, Hetzel MR. Role of the neodymium yag laser in the management of tracheal tumours. Thorax 1987;42:440-4. [Crossref] [PubMed]
- Parr GV, Unger M, Trout RG, et al. One hundred neodymium-yag laser ablations of obstructing tracheal neoplasms. Ann Thorac Surg 1984;38:374-81. [Crossref] [PubMed]
- Choi TK, Siu KF, Lam KH, et al. Bronchoscopy and carcinoma of the esophagus ii. Carcinoma of the esophagus with tracheobronchial involvement. Am J Surg 1984;147:760-2. [Crossref] [PubMed]
- Matsubara T, Ueda M, Nagao N, et al. Surgical treatment for carcinoma of the thoracic esophagus with major involvement in the neck or upper mediastinum. J Surg Oncol 1998;67:6-10. [Crossref] [PubMed]
- Gaafar AH, Shaaban AY, Elhadidi MS. The use of metallic expandable tracheal stents in the management of inoperable malignant tracheal obstruction. Eur Arch Otorhinolaryngol 2012;269:247-53. [Crossref] [PubMed]
- Vishwanath G, Madan K, Bal A, et al. Rigid bronchoscopy and mechanical debulking in the management of central airway tumors: An indian experience. J Bronchology Interv Pulmonol 2013;20:127-33. [Crossref] [PubMed]
- Dalar L, Ozdemir C, Sokucu SN, et al. Bronchoscopic palliation to treat endobronchial metastasis of the tracheobronchial tree. Respir Investig 2016;54:116-20. [Crossref] [PubMed]
- Nakao K, Miyata M, Izukura M, et al. Radical operation for thyroid carcinoma invading the trachea. Arch Surg 1984;119:1046-9. [Crossref] [PubMed]
- Ito Y, Fukushima M, Yabuta T, et al. Local prognosis of patients with papillary thyroid carcinoma who were intra-operatively diagnosed as having minimal invasion of the trachea: A 17-year experience in a single institute. Asian J Surg 2009;32:102-8. [Crossref] [PubMed]
- Lee DY, Won JK, Choi HS, et al. Recurrence and survival after gross total removal of resectable undifferentiated or poorly differentiated thyroid carcinoma. Thyroid 2016;26:1259-68. [Crossref] [PubMed]
- Kim BY, Choi JE, Lee E, et al. Prognostic factors for recurrence of locally advanced differentiated thyroid cancer. J Surg Oncol 2017;116:877-83. [Crossref] [PubMed]
- Seeburger JL, Stepak M, Fukuchi SG, et al. Multiple arterial thromboembolisms in a patient with the 20210 a prothrombin gene mutation. Arch Surg 2000;135:721-2. [Crossref] [PubMed]
- Honings J, Stephen AE, Marres HA, et al. The management of thyroid carcinoma invading the larynx or trachea. Laryngoscope 2010;120:682-9. [Crossref] [PubMed]
- Tytor M, Olofsson J. Thyroid tumors invading the larynx and trachea. J Otolaryngol 1986;15:74-9. [PubMed]
- Ribechini A, Bottici V, Chella A, et al. Interventional bronchoscopy in the treatment of tracheal obstruction secondary to advanced thyroid cancer. J Endocrinol Invest 2006;29:131-5. [Crossref] [PubMed]
- Noppen M, Poppe K, D'Haese J, et al. Interventional bronchoscopy for treatment of tracheal obstruction secondary to benign or malignant thyroid disease. Chest 2004;125:723-30. [Crossref] [PubMed]
- Leung AK, Chow SM, Law SC. Clinical features and outcome of the tall cell variant of papillary thyroid carcinoma. Laryngoscope 2008;118:32-8. [Crossref] [PubMed]
- Bishop JA, Wu G, Tufano RP, et al. Histological patterns of locoregional recurrence in hurthle cell carcinoma of the thyroid gland. Thyroid 2012;22:690-4. [Crossref] [PubMed]
- Yalçin B, Demir HA, Ciftci AO, et al. Thymomas in childhood: 11 cases from a single institution. J Pediatr Hematol Oncol 2012;34:601-5. [Crossref] [PubMed]
- Riedel M, Stein HJ, Mounyam L, et al. Predictors of tracheobronchial invasion of suprabifurcal oesophageal cancer. Respiration 2000;67:630-7. [Crossref] [PubMed]
- Mukai T, Joh K, Arai Y, et al. Tissue-specific expression of rat aldolase a mrnas. Three molecular species differing only in the 5'-terminal sequences. J Biol Chem 1986;261:3347-54. [PubMed]
- Sharpe DA, Dixon K, Moghissi K. Endoscopic laser treatment for tracheal obstruction. Eur J Cardiothorac Surg 1996;10:722-6. [Crossref] [PubMed]
- Shapshay SM, Strong MS. Tracheobronchial obstruction from metastatic distant malignancies. Ann Otol Rhinol Laryngol 1982;91:648-51. [Crossref] [PubMed]
- Maeda M, Nakamoto K, Ohta M, et al. Statistical survey of tracheobronchoplasty in japan. J Thorac Cardiovasc Surg 1989;97:402-14. [PubMed]
- Nakahira M, Nakatani H, Takeuchi S, et al. Safe reconstruction of a large cervico-mediastinal tracheal defect with a pectoralis major myocutaneous flap and free costal cartilage grafts. Auris Nasus Larynx 2006;33:203-6. [Crossref] [PubMed]
- Fabre D, Kolb F, Fadel E, et al. Successful tracheal replacement in humans using autologous tissues: An 8-year experience. Ann Thorac Surg 2013;96:1146-55. [Crossref] [PubMed]
- Maciejewski A, Szymczyk C, Poltorak S, et al. Tracheal reconstruction with the use of radial forearm free flap combined with biodegradative mesh suspension. Ann Thorac Surg 2009;87:608-10. [Crossref] [PubMed]
- Zerner J. Metastatic carcinoma (endometrial adenoacanthoma) to the trachea. Report of a successful resection and primary anastomosis. J Thorac Cardiovasc Surg 1975;70:139-42. [PubMed]
- Andrews AH Jr, Caldarelli DD. Carbon dioxide laser treatment of metastatic melanoma of the trachea and bronchi. Ann Otol Rhinol Laryngol 1981;90:310-1. [Crossref] [PubMed]
- Koyi H, Branden E. Intratracheal metastasis from malignant melanoma. J Eur Acad Dermatol Venereol 2000;14:407-8. [Crossref] [PubMed]
- Cooper JA Jr, Kapp DS, Swett HA, et al. Acute bilobar collapse secondary to endobronchial metastatic seminoma. J Can Assoc Radiol 1985;36:166-7. [PubMed]