Cerebral embolic protection during endovascular arch replacement
Introduction
One of the most devastating complications after cardiac surgery is stroke. Conventional open repair of aortic arch pathology is highly invasive and frequently requires extracorporeal circulation and hypothermic circulatory arrest, both sources of stroke. Despite advances in perioperative management and surgical technique, contemporary series of aortic arch repair report a significant risk of stroke (4.7–6.0%) (1,2).
Near-total endovascular repair of aortic arch pathology, and hybrid approaches that combine surgical revascularization of the brachiocephalic arteries with thoracic endovascular aortic repair (TEVAR) have appeared as promising alternatives. Although these approaches can mitigate risk associated with cardiopulmonary bypass, circulatory arrest, and aortic cross clamping, strokes after endovascular procedures of the arch still contribute a significant risk to the patient with rates of 4.2–5.9% (Table 1) (3-5). Mechanisms of stroke include embolism or aortic dissection with wire and catheter manipulations, and during deployments of endografts, in addition to cerebral hypoperfusion during revascularization of the supra-aortic trunks. Sources of emboli may include fresh or organized thrombus, atheromatous debris, air, or native arterial tissue. Several strategies exist to mitigate stroke risk during endovascular approaches to the arch. However, new innovations in device design constantly introduce new challenges in stroke reduction.
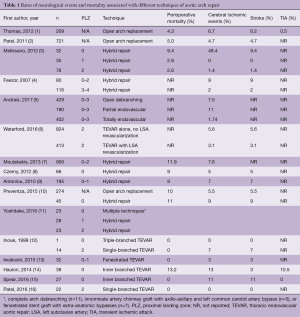
Full table
Epidemiology of stroke in arch endovascular procedures
Stroke risk during arch TEVAR varies with both the method and extent of repair. With rapidly developing endovascular technology for treatment of arch pathology, elucidating rates and causes of peri-operative stroke has been difficult. Challenging arch anatomy, advancing devices and wires proximally into the aortic arch and ascending aorta, and complexity of the procedure all contribute to the differences in stroke rates. A greater understanding of the epidemiology of stroke can assist in anticipation and avoidance of stroke in endovascular approaches to arch pathology.
Atheromatous disease
Aortic arch atherosclerosis is commonly found in the population and can contribute to embolic phenomena. In the SPARC study, 51% of the population over the age of ≥45 years had the presence of arch atheroma with 7.6% having severe atheroma as defined by ≥4 mm thick, ulcerated or mobile plaque (17). The presence of severe arch atheroma increases with age and is found in over 20% of patients ≥75 years.
Several studies of TEVAR for distal arch or descending aortic pathology have demonstrated an association of significant arch atheroma/shaggy aorta and peripheral embolization with stroke (18-21).
Hybrid approaches
When hybrid arch repair involves proximal arch landing zones, surgical debranching is required to maintain cerebral perfusion when the endograft is expected to exclude the supra-aortic vessels. Hybrid approaches have the benefit of combining a more limited, less invasive open revascularization with a relatively straightforward endovascular procedure. Although many configurations of hybrid approaches exist, the University of Pennsylvania group classified hybrid approaches into a practical classification that includes three types of repair (Figure 1) (22). Type I includes aortic debranching using a partial aortic clamp and a zone 0 landing zone, completely avoiding cardiopulmonary bypass. Type II requires surgical construction of a zone 0 landing zone requiring cardiopulmonary bypass and often circulatory arrest. Type III hybrid approaches include full reconstruction of the ascending aorta and arch. Further expansion of this classification can include arch reconstruction at zone 1 or 2. Selected strategies are tailored to patient anatomy.
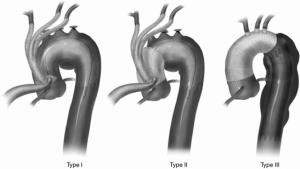
Stroke rates in hybrid repairs are influenced by cross-clamping, clamping of branches vessels, hypoperfusion, circulatory arrest, and endovascular manipulations.
Major strokes occurred during hybrid repair for zone 0 or zone 1 pathology in 4.7–9.5% (11) of patients (3,6-10). Although robust data for stroke rates at each zone is sparse, higher stroke rates seem to correspond with more proximal debranching with zone 0, zone 1, and zone 2 hybrid repair stroke rates being 17.4%, 7.1%, and 4.3%, respectively (11).
Procedures with increased wire manipulation
Repair with fenestrated/branched endografts
Fenestrated and branched endografts for the aortic arch were developed as a means to achieve near total endovascular repair of the arch, eliminating most of the debranching required with hybrid repair. The majority of the global experience with branched arch endografts is with the Cook Medical Zenith arch branched graft (Bloomington, IN, USA), a two-branch device developed for proximal sealing in zone 0. All patients undergo left subclavian artery (LSA) revascularization and the branches are bridged to their target vessels (innominate and left common carotid) with an appropriate covered bridging stent graft. The initial global experience of 38 patients treated with this device was reported in 2014 in patient who were deemed high-risk or unfit for conventional surgery. Stroke, transient ischemic attack (TIA) and meningeal hemorrhage occurred in six patients (15.7%) (14). A more recent study using the same device in high risk patients reported two major strokes among 27 patients treated (7.4%) (15). The authors concluded that decreased stroke rate was directly correlated with the learning curve involved with these complex novel procedures.
Arch endografts with multiple fenestrations or branches [Nexus (Endospan Ltd., Herzlia, Israel); arch branch graft (Cook Medical); arch branched stent graft (Bolton Medical Inc., Sunrise, Florida, USA)] (Figure 2) are not currently commercially available in the United States, and are generally only available worldwide under special or compassionate use access although plans are underway for feasibility clinical trials in the United States the Gore TAG Thoracic Branch Endoprosthesis (W.L. Gore, Flagstaff, AZ, USA), a single branch device (Figure 2), is currently undergoing a US pivotal clinical trial. While this pivotal trial has recently been opened up to zone 0 and 1 pathology, the initial results of repair in zone 2 (Gore TAG TBE feasibility trial) were published, demonstrating no strokes in 22 patients treated (16). Medtronic has recently restarted their feasibility trial examining safety and efficacy of their Mona LSA branch stent graft system, with a single “Volcano” like branch intended for zone 2 cases (Figure 2). The high-risk nature of the patients, increased wire and catheter manipulation in the branch vessels and arch, and deployment of branch devices and balloons in the branch vessels all contribute to stroke risk in these patients.
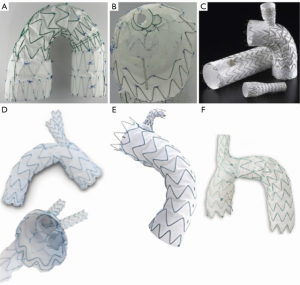
Repair with parallel grafts
Parallel chimney stents were initially conceived as a bailout maneuver in cases of inadvertent visceral artery coverage during endovascular abdominal aortic repair, but the technique has since been extended to other applications (23). It has been used widely in the paravisceral aorta and is increasingly being used to manage the supra-aortic branches during TEVAR. Briefly, a covered stent runs in parallel to the aortic endoprosthesis into the branch vessel and serves as a bridge from the aorta, within the seal zone, to the vessel that has been intentionally covered. The principal advantage of this off label technique is in its use of off-the-shelf components and techniques familiar to most operators to achieve a more proximal landing zone during arch TEVAR. The disadvantage of this technique is the creation of gutters around the aortic endoprosthesis with a 6.4–9.4% rate of type IA endoleak (24,25).
The rate of major stroke during chimney TEVAR was 2.6–5.4% (20,25). In Hogendoorn et al., 24% of patients who underwent arch chimney grafts required an additional cervical debranching procedure; the stroke rate in these patients was 8.3% versus 3.2% for those who did not have additional revascularization, with 40% of strokes being fatal (25).
Implantation of chimney grafts requires more wire and catheter manipulations within the arch compared to TEVAR with debranching, and these manipulations in and around the arch vessels may contribute to cerebral embolization.
LSA coverage
In order to achieve a more stable and reliable proximal landing zone, or as part of proximal arch procedures, the LSA will often require endovascular occlusion. Stroke rates of 4.8–7.4% have been reported in patients undergoing TEVAR with LSA coverage (6). The Eurostar registry has identified LSA coverage as significantly associated with higher neurological sequelae (26). Coverage of the LSA with and without revascularization demonstrated lower risk of stroke in patients who had LSA revascularization (5.3% 95% CI: 2.6–8.6 vs. 8.0% 95% CI: 4.1–12.4) (17); and 3.1% vs. 5.6%, P=0.0657 (6). We recommend LSA revascularization whenever feasible.
Techniques at minimizing stroke
Pre-operative planning
The planning phase of arch endovascular therapy remains an essential step in stroke minimization. Anticipating and avoiding potential sources of stroke is likely the best strategy in stroke prevention.
Imaging
Identifying potential embolic sources on chest CTA imaging can alter pre-operative planning as well as intra-operative strategies. Calcific disease, atheroma, dissection flaps, and ulcerations can all contribute to embolic phenomena and strategies aimed at avoiding or excluding these zones from circulation are ideal. Knowledge of the presence and dominance of vertebral artery circulation is required for subclavian artery revascularization options, and head and neck imaging are strongly recommended to establish circle of Willis anatomy. Branch origin disease, tortuosity, and arch elongation can contribute to technical challenges in advancing and positioning devices in the ascending aorta, branch vessels, and arch. Anticipating these challenges can minimize wire manipulations and failed attempts at advancing devices, thus reducing risk of stroke.
ECG-gated computed tomography angiography is the ideal imaging modality for arch planning. Gating, whether prospective ECG triggered or retrospective ECG, improves temporal resolution and minimizes artifacts caused by cardiac motion (27) allowing for accuracy in measurement. Uploading images into 3D software for measurement is highly recommended.
Device selection
The anatomical constraints of the ascending aorta and arch can be extremely unforgiving in the endovascular setting. Arch configuration and aortic tortuosity (proximal or distal) can contribute to challenges in advancing wires, catheters, and devices without ‘snowplowing’ the aortic wall. When these situations are encountered, the most important step in stroke minimization is selecting the proper approach to managing the aortic pathology. In highly angulated or elongated arches, total arch endovascular techniques may carry high stroke risk compared to hybrid techniques and when atheromatous burden is high endovascular manipulations in the high-risk arch may be better avoided, preferring a completely open strategy. When selecting which device or strategy to employ for arch pathology, device conformability, stiffness of the delivery system, and operator familiarity should all be considered.
Intra-operative techniques
Wires and catheters
Proper wire and catheter use represents best clinical practice in stroke reduction. Prior to advancing any wires across the arch, adequate heparinization with 100–200 units/kg of Heparin with a goal ACT of >250 must be achieved. Furthermore, it is recommended that American Society of Anesthesiologists Physical Status Classification System (ASA) and, selectively, clopidogrel be continued throughout the procedure.
Wires should never be advanced without fluoroscopic visualization. Whenever possible, standardizing wire and catheter selection to minimize unnecessary exchanges is advisable. The decision to position the wire in the ascending aorta or in the left ventricle, ideally should be determined pre-procedurally. Careful attention to marking and stabilizing these wires is essential. If advanced wire techniques are necessary such as through and through wire access, snaring should be performed distal to the arch to avoid inadvertent cerebral embolization.
Device manipulation
When anatomical constraints present challenges in device delivery or deployment, the operator must move quickly to alternate techniques, abandoning multiple or forced ineffective attempts. Stiff buddy wires and through and through wire access (brachial/subclavian/axillary) can provide the support needed to navigate tortuous anatomy and safely advance delivery systems into the proximal aorta. These techniques can be planned in challenging anatomy. Iliac and aortic tortuosity is often underestimated as a possible cause of inability to introduce the device into the arch. Pre-planning is often beneficial to countering these challenges and alternative access options such as iliac conduits or direct abdominal aortic access can circumvent these obstacles.
Adjunctive procedures and devices
LSA revascularization
When feasible, we strongly recommend routine carotid-subclavian bypass or transposition of the LSA, in order to minimize stroke and spinal injury. If lengthy endovascular procedures are planned, LSA revascularization is performed in a staged fashion the day prior.
Embolic protection devices (EPD)
There is a paucity of data regarding techniques or devices for embolic protection for use during arch TEVAR. Experience with methods for prevention of embolization has been gained in other endovascular procedures at risk for cerebral embolization, specifically in internal carotid artery stenting (CAS) and transcatheter aortic valve replacement (TAVR).
The most commonly used EPD are distal filters with occlusion/aspiration systems used less commonly (28). Distal filter EPDs are deployed over a 0.014" wire or come attached to a steerable wire tip and allow antegrade carotid blood flow during the entirety of the procedure, real-time capture of embolic debris, and angiography at any time during the intervention. In CAS, use of EPDs has demonstrated a significant reduction in both minor stroke (0.5% vs. 3.7%; P<0.001) and major stroke (0.3% vs. 1.1%; P<0.05) (29) and are currently in widespread use for this application.
Various EPDs have been studied in the setting of TAVR. The Claret Sentinel device (Claret Medical Inc., Santa Rosa, California, USA) consists of a dual-filter system inserted through a 6 French sheath inserted via right arm access. The proximal component, a radiopaque nitinol frame with a 140-µm pore polyurethane filter, is deployed in the IA and a second filter is positioned across the left common carotid artery (LCCA) ostium. This device protects the right vertebral, right carotid, and left carotid arteries from embolization but not the left vertebral. Conversely, the TriGuard HDH embolic deflection device (Keystone Heart Ltd., Caesarea, IL, USA) is delivered transfemorally via a 9 French Mullins introducer sheath and deploys a single mesh filter with 130-µm pores across the ostia of all three head vessels. These devices demonstrated significantly decreased risk of stroke in patients undergoing TAVR procedures (30,31). Disadvantages of these EPDs include the requirement of an additional arterial access (up to 9 French in the case of the TriGuard), lack of protection of the left vertebral artery by the Sentinel device, and availability of only one size for each device leading to incomplete coverage in certain arch anatomies. Variability in arch anatomy and the wide spectrum of possible arch pathologies makes no single embolic protection device or technique applicable to all situations or repair techniques. Diversion/filtering devices used for TAVR pose a technical challenge for embolic protection during zone 0–2 arch repair compared to when they are used during TAVR as they are likely to interfere with the deployment of the aortic endoprosthesis. If still in place once the TEVAR is implanted, removal of the EPD could potentially displace the endoprosthesis or more importantly result in entrapment of the EPD. Two additional EPD devices, the Embol-X and Embrella devices did not demonstrate reduction in the incidence of large total lesion volume. The Embol-X device study was stopped before completion due to commercial unavailability of the device, and studies of the Embrella device were not powered for efficacy, and actually trended towards an increase in lesion numbers (32). Neither of these devices are commercially available in the United States (12).
The senior author has used the Boston Scientific FilterWire EZ (Figure 3) EPD in four cases of advanced aortic arch intervention. These devices are intended for transfemoral placement into the mid internal carotid artery. This method of use is contraindicated for arch branched cases due to the device being entrapped by the full deployment of the arch branched graft. Therefore, if one intends to use these neuroprotective devices, they must be placed via direct access of the supra-aortic vessels. On the right, direct mid to distal common carotid access is required, and for left internal carotid deployment, the device is introduced by way of the left carotid subclavian bypass usually by direct puncture in the synthetic graft. Direct access as described above can be cumbersome and awkward with the long delivery systems of the EPD being exposed to possible inadvertent manipulations and credible fear that the EPD could be dragged back on an exposed internal carotid artery. Even more worrisome is the concern for the 0.014" delivery wire being bent or kinked while distracted by the other manipulations of the arch branched graft or the supra aortic access. Clearly, if carotid neuroprotection is desired using this modality, development of a more procedure-specific neuroprotection device is required. Interestingly, despite the use of direct neuroprotection with EPD in the internal carotid artery, one out of these four patients experienced a significant stroke. Examination of the filter in that case demonstrated significant debris in the filter, confirming the very real possibility of embolic potential in these complex arch cases (Figure 4).


Carotid clamping
Clamping and flushing of the carotid vessels is a relatively simple method of embolic protection. It can be considered when stroke risk is high secondary to excessive atheromatous burden in the ascending aorta and arch. This technique is limited by a few different variables: not all vessels are surgically exposed in every case; clamping of the artery itself may cause embolization in the cases of diseased arteries; and cerebral hypoperfusion can occur. In general, surgeons experienced with clamping currently favor the technique of sequential clamping of the common carotid artery distal to the retrograde catheterization point while the branch intervention is performed, with subsequent flushing before opening the carotid to antegrade flow. This of course is predicated by adequate collateral flow via a patent circle of Willis, along with systemic blood pressure that supports this robust cerebral network. We routinely maintain systemic blood pressure in the normotensive or hypertensive during range during carotid clamping to minimize the risk of hypoperfusion.
Conclusions
Many factors can contribute to the risk of stroke during endovascular therapy to arch pathology. Approaches to the arch whether open, near-total endovascular, or hybrid, all carry different risk of stroke but must be individualized to the patient in order to achieve the best outcomes with the lowest risk of peri-operative mortality and stroke. Patient factors such as arch atheroma and ulceration, and arch elongation, are not modifiable and must be identified early and be strategically considered during the planning phase. Currently, there are no neuroprotection devices available that are specifically compatible with endovascular repair of arch pathology. Techniques in stroke prevention such as carotid clamping and/or EPD placed directly through supra-aortic vessels should be considered when appropriate, on an individual basis. Sound clinical practice such as diligence with wire and device manipulations is always recommended. As endovascular therapy continues to push the boundaries of aortic arch management, the cause and prevention of strokes will continue to evolve. There is clearly opportunity and need for innovation in neuroprotection for complex endovascular arch intervention. Until this need is met, stroke will continue to be the Achilles heel of endovascular aortic arch intervention.
Acknowledgements
None.
Footnote
Conflicts of Interest: Dr Cherrie Abraham is a Consultant for Cook Medical (Case reviews, Proctoring Advanced Aortic Intervention), Medtronic (Aortic Advisory Board), and WL Gore (Clinical Events Committee, Gore Clinical Trial) -- (Consultant fees paid to OHSU). The other authors have no conflicts of interest to declare.
References
- Thomas M, Li Z, Cook DJ, et al. Contemporary results of open aortic arch surgery. J Thorac Cardiovasc Surg 2012;144:838-44. [Crossref] [PubMed]
- Patel HJ, Nguyen C, Diener AC, et al. Open arch reconstruction in the endovascular era: analysis of 721 patients over 17 years. J Thorac Cardiovasc Surg 2011;141:1417-23. [Crossref] [PubMed]
- Melissano G, Tshomba Y, Bertoglio L, et al. Analysis of stroke after TEVAR involving the aortic arch. Eur J Vasc Endovasc Surg 2012;43:269-75. [Crossref] [PubMed]
- Feezor RJ, Martin TD, Hess PJ, et al. Risk factors for perioperative stroke during thoracic endovascular aortic repairs (TEVAR). J Endovasc Ther 2007;14:568-73. [Crossref] [PubMed]
- Andrási TB, Grossmann M, Zenker D, et al. Supra-aortic interventions for endovascular exclusion of the entire aortic arch. J Vasc Surg 2017;66:281-97.e2. [Crossref] [PubMed]
- Waterford SD, Chou D, Bombien R, et al. Left Subclavian Arterial Coverage and Stroke During Thoracic Aortic Endografting: A Systematic Review. Ann Thorac Surg 2016;101:381-9. [Crossref] [PubMed]
- Moulakakis KG, Mylonas SN, Markatis F, et al. A systematic review and meta-analysis of hybrid aortic arch replacement. Ann Cardiothorac Surg 2013;2:247-60. [PubMed]
- Czerny M, Weigang E, Sodeck G, et al. Targeting landing zone 0 by total arch rerouting and TEVAR: midterm results of a transcontinental registry. Ann Thorac Surg 2012;94:84-9. [Crossref] [PubMed]
- Antoniou GA, El Sakka K, Hamady M, et al. Hybrid treatment of complex aortic arch disease with supra-aortic debranching and endovascular stent graft repair. Eur J Vasc Endovasc Surg 2010;39:683-90. [Crossref] [PubMed]
- Preventza O, Garcia A, Cooley DA, et al. Total aortic arch replacement: A comparative study of zone 0 hybrid arch exclusion versus traditional open repair. J Thorac Cardiovasc Surg 2015;150:1591-8; discussion 1598-600. [Crossref] [PubMed]
- Yoshitake A, Hachiya T, Okamoto K, et al. Postoperative Stroke after Debranching with Thoracic Endovascular Aortic Repair. Ann Vasc Surg 2016;36:132-8. [Crossref] [PubMed]
- Inoue K, Hosokawa H, Iwase T, et al. Aortic arch reconstruction by transluminally placed endovascular branched stent graft. Circulation 1999;100:II316-21. [Crossref] [PubMed]
- Iwakoshi S, Ichihashi S, Itoh H, et al. Clinical outcomes of thoracic endovascular aneurysm repair using commercially available fenestrated stent graft (Najuta endograft). J Vasc Surg 2015;62:1473-8. [Crossref] [PubMed]
- Haulon S, Greenberg RK, Spear R, et al. Global experience with an inner branched arch endograft. J Thorac Cardiovasc Surg 2014;148:1709-16. [Crossref] [PubMed]
- Spear R, Haulon S, Ohki T, et al. Editor's Choice - Subsequent Results for Arch Aneurysm Repair with Inner Branched Endografts. Eur J Vasc Endovasc Surg 2016;51:380-5. [Crossref] [PubMed]
- Patel HJ, Dake MD, Bavaria JE, et al. Branched Endovascular Therapy of the Distal Aortic Arch: Preliminary Results of the Feasibility Multicenter Trial of the Gore Thoracic Branch Endoprosthesis. Ann Thorac Surg 2016;102:1190-8. [Crossref] [PubMed]
- Macleod MR, Amarenco P, Davis SM, et al. Atheroma of the aortic arch: an important and poorly recognised factor in the aetiology of stroke. Lancet 2004;3:408-14. [Crossref] [PubMed]
- Kotelis D, Bischoff MS, Jobst B, et al. Morphological risk factors of stroke during thoracic endovascular aortic repair. Langenbecks Arch Surg 2012;397:1267-73. [Crossref] [PubMed]
- Gutsche JT, Cheung AT, McGarvery ML, et al. Risk factors for perioperative stroke after thoracic endovascular aortic repair. Ann Thorac Surg 2007;84:1195-200. [Crossref] [PubMed]
- Khoynezhad A, Donayre CE, Bui H, et al. Risk factors of neurologic deficit after thoracic aortic endografting. Ann Thorac Surg 2007;83:S882-9. [Crossref] [PubMed]
- Kanaoka Y, Ohki T, Maeda K, et al. Multivariate Analysis of Risk Factors of Cerebral Infarction in 439 Patients Undergoing Thoracic Endovascular Aneurysm Repair. Medicine (Baltimore) 2016;95:e3335. [Crossref] [PubMed]
- Vallabhajoysula P, Bavaria JE, Szeto WY. Hybrid Approaches to Complex Aortic Arch Aneurysms. J Thorac Cardiovasc Surg 2012;17:15-26.
- Voskresensky I, Scali ST, Feezor RJ, et al. Outcomes of thoracic endovascular aortic repair using aortic arch chimney stents in high-risk patients. J Vasc Surg 2017;66:9-20.e3. [Crossref] [PubMed]
- Ahmad W, Mylonas S, Majd P, et al. A current systematic evaluation and meta-analysis of chimney graft technology in aortic arch diseases. J Vasc Surg 2017;66:1602-10.e2. [Crossref] [PubMed]
- Hogendoorn W, Schlösser FJV, Moll FL, et al. Thoracic endovascular aortic repair with the chimney graft technique. J Vasc Surg 2013;58:502-11. [Crossref] [PubMed]
- Buth J, Harris PL, Hobo R, et al. Neurologic complications associated with endovascular repair of thoracic aortic pathology: incidence and risk factors. A study from the European collaborators on stent/graft techniques for aortic aneurysm repair (EUROSTAR) registry. J Vasc Surg 2007;46:1103-10. [Crossref] [PubMed]
- Desjardins B, Kazerooni EA. ECG Gated Cardiac CT. AJR Am J Roentgenol 2004;182:993-1010. [Crossref] [PubMed]
- Mousa AY, Campbell JE, AbuRahma AF, et al. Current update of cerebral embolic protection devices. J Vasc Surg 2012;56:1429-37. [Crossref] [PubMed]
- Kastrup A, Gröschel K, Krapf H, et al. Early outcome of carotid angioplasty and stenting with and without cerebral protection devices: a systematic review of the literature. Stroke 2003;34:813-9. [Crossref] [PubMed]
- Giustino G, Sorrentino S, Mehran R, et al. Cerebral Embolic Protection During TAVR: A Clinical Event Meta-Analysis. J Am Coll Cardiol 2017;69:465-6. [Crossref] [PubMed]
- Seeger J, Gonska B, Otto M, et al. Cerebral Embolic Protection During Transcatheter Aortic Valve Replacement Significantly Reduces Death and Stroke Compared With Unprotected Procedures. JACC Cardiovasc Interv 2017;10:2297-303. [Crossref] [PubMed]
- Vlastra W, Vendrik J, Koch KT, et al. Cerebral protection devices during transcatheter aortic valve implantation. Trends in Cardiovascular Medicine 2018. In Press. [Crossref] [PubMed]





