Early clinical results with the Tendyne transcatheter mitral valve replacement system
Introduction
Mitral valve regurgitation (MR) is a leading cause of valvular heart disease (1). As the population ages, the burden of this disease is expected to increase. However, only a small proportion of those affected are referred for surgical correction (2). The basis for this under-treatment is multifactorial; commonly acknowledged factors include an underestimation of the risk posed by MR and concerns regarding the excess risk associated with conventional surgery (3,4). In addition, the benefits of surgery for functional mitral regurgitation are not clear, with only a class IIb recommendation for surgical intervention (5). Given this landscape, there has been considerable interest in the development of transcatheter mitral valve systems with dozens of devices in various stages of development (6).
Device components
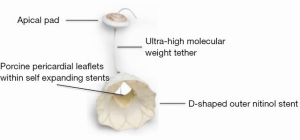
The Tendyne (Tendyne Holdings, LLC, Roseville, Minnesota—a subsidiary of Abbott Vascular) bioprosthetic mitral valve system is a fully repositionable and retrievable transcatheter mitral valve replacement (TMVR) device (7,8). It is delivered through a 34-Fr transapical sheath, accessed via a small left anterior thoracotomy. The valve itself is composed of three porcine pericardial leaflets that are sewn into two self-expanding nitinol stents (Figure 1). The Tendyne TMVR is the only mitral valve system that has three available sizes. However, the inner stent is the same for all three sizes maintaining an effective orifice area of at least 3.2 cm2. The outer stent is designed to match the D-shape of the native mitral annulus, with the straight edge of the cuff resting along the atrial wall on the aortomitral continuity. Attached to the prosthesis is an apical tether made of ultra-high molecular weight polyethylene. This fiber is secured to a pad that rests on the apical epicardial surface.
Keys to successful device implantation—preoperative planning
Careful preoperative planning and patient selection is imperative for successful deployment of the valve. A gated, contrast enhanced, cardiac computed tomography is used to assess valve geometry and relationship to left ventricular structures. Specific consideration should be given to the septal-lateral diameter, intercommissural diameter, aorto-mitral angle, anterior mitral valve leaflet length and left ventricular outflow tract (LVOT) dimensions (9). Various device sizes can be implanted into an annulus with a septal-lateral diameter between 30–43 mm and those with an intercommissural range of 34–50 mm. Evaluation of preoperative imaging also enables determination of the ideal location for apical access in order to allow for a coaxial approach to the mitral valve (10). Finally, the risk of LVOT obstruction should be evaluated using these measures alongside a simulated virtual valve implantation with multiplanar reformatting (11).
Keys to successful device implantation—procedural considerations
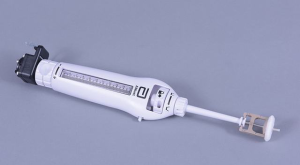
The apex of the heart is visualized after a small left anterior thoracotomy is made. A combination of digital palpation and review of preoperative imaging helps to confirm the ideal location for transapical access. Optimal positioning of the transapical access is necessary, as this determines the location of the apical tether mechanism. Careful selection minimizes the risk of paravalvular leak and outflow tract obstruction. Pledgeted purse string sutures are placed in the left ventricular apex and the 34-Fr sheath is inserted after obtaining left ventricular and left atrial access. It is critical to ensure that the mitral subvalvular apparatus is not entangled. Next, the Tendyne valve is inserted through the sheath into the left atrium. The valve is then partially deployed to allow orientation under transesophageal echocardiographic guidance. The valve is then fully opened and pulled into the annulus. Finally, the tether is secured to the apical pad with a tensioning tool (Figure 2) in order to minimize paravalvular leak, prevent device migration and to aid hemostasis of the left ventricular access site.
Clinical results
The first-in-human implantation occurred in February 2013 and was reported as a two-patient series the following year (12). In both patients, the valve system was deployed with dramatic improvement in the grade of MR and intracardiac pressures. As dictated by the study protocol, after deployment, the devices were explanted and the patients went on to conventional surgical valve replacement surgery.
Following this initial experience, three additional patients underwent successful implantation in late 2014 through a compassionate use protocol in the United Kingdom (13). All patients reported significant symptomatic improvement at 30 days. In addition, there was improvement in tricuspid regurgitation and pulmonary hypertension in all patients. Subsequently, the same group published their long-term outcomes from these initial procedures and included an additional two patients that were implanted over the months that followed (9). There were no procedural deaths, but one patient developed LVOT obstruction that required stenting of the LVOT. In retrospect, the patient was identified as high risk for LVOT obstruction due to her relatively long anterior mitral valve leaflet (2.75 cm) and small LVOT diameter (1.7 cm). At 1 year, 1 of 5 patients had died due to complications related to a cerebrovascular accident in the context of anti-thrombotic non-compliance. At the latest available follow-up of 18–24 months, all living patients reported reduction in New York Heart Association (NYHA) class and stable device function.
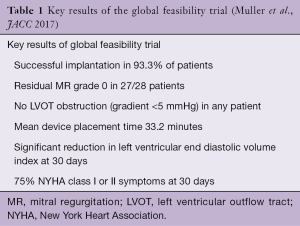
The largest published experience with this device comes from the global feasibility trial, which enrolled 30 patients at 8 sites between 2014–2016 (7). This remains one of the largest studies evaluating TMVR technology to date (14). High surgical risk patients with grade 3 or 4 (primary or secondary) MR and NYHA functional class ≥ II were enrolled. Overall, the average Society for Thoracic Surgeons (STS) predicted risk of mortality for surgical mitral valve replacement was 7.3%. The majority had at least a component of secondary MR (90%) and more than half had reduced ejection fractions of less than 50% (58.6%). The device was successfully deployed in 93% of patients. For those with successful deployment, one patient had mild (1+) residual MR while there was no residual MR in all others (Table 1). No patients with device implantation developed a LVOT gradient. At 30 days, there was a significant improvement in NYHA functional class. Mild or no symptoms (NYHA I or II) were reported in 75% of patients, as compared to 47% (all NYHA II) at baseline. The procedural reduction in MR persisted at 30 days, with one patient having mild central MR and all others without MR. Successful implantation with freedom from cardiovascular death, stroke and device dysfunction at 30 days was 87.0%.
Full table
In May 2018, the results of the first 100 patients enrolled in the international, CE mark study were presented at the Euro PCR conference. This non-randomized cohort enrolled patients that were deemed poor surgical candidates by the Heart Team and predominantly included patients with secondary MR. The mean STS predicted risk of mortality for surgical mitral valve replacement was 7.9%. Nearly all devices (97%) were implanted successfully, with no procedural mortality, surgical conversion, or need for mechanical circulatory support. Of the 76 patients with complete 30-day data, 98.7% had no or trace MR at 30 days. In paired analyses, the percentage of patients with NYHA class III or IV symptoms decreased from baseline (62.5% vs. 24.9%, P<0.0001), while Kansas City Cardiomyopathy Questionnaire scores showed significant improvement from baseline (50.5 vs. 58, P=0.037).
Future challenges and unanswered questions
On the heels of a successful feasibility trial and the promising preliminary results of the CE mark study, a number of questions will need to be answered before widespread application in clinical practice is seen. More rigorous study design with either medically treated controls or alternative intervention arms are necessary to understand the benefit of this device. These studies will help to define the true incidence of valve related complications such as thrombosis, migration and access site complications. In addition, given the heterogeneity of mitral valve pathology and the range of devices currently under clinical investigation, matching individual patients with appropriate devices will evolve as a new and increasingly complex multidisciplinary challenge (15). The role of the heart team will continue to expand as more options are added to this nuanced algorithm. Ongoing and planned clinical trials will help to answer remaining uncertainties surrounding this platform, including the SUMMIT trial, which recently began enrolling patients.
In conclusion, the Tendyne system has demonstrated safety and early efficacy in a small group of selected patients. The high rate of successful deployment, few procedural complications and device stability should be regarded with enthusiasm. Although the device remains in the early stages of clinical investigation, it has emerged as an exciting and important developing technology in mitral valve surgery.
Acknowledgements
Funding: This work was supported by the National Heart, Lung, and Blood Institute (grant No. T32 HL007849).
Footnote
Conflicts of Interest: Dr. G Ailawadi is a consultant for Abbott, Edwards Lifesciences, Medtronic, Mitralign, and Cephea. Dr. VH Thourani is a consultant for Abbott, Boston Scientific, Edwards Lifesciences, and Gore Vascular. Dr. JH Rogers is a consultant for Tendyne Holdings, Millipede, Boston Scientific, and Pipeline. JP Beller has no conflicts of interest to declare.
References
- Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005-11. [Crossref] [PubMed]
- Dziadzko V, Clavel MA, Dziadzko M, et al. Outcome and undertreatment of mitral regurgitation: a community cohort study. Lancet 2018;391:960-9. [Crossref] [PubMed]
- Bach DS, Awais M, Gurm HS, et al. Failure of guideline adherence for intervention in patients with severe mitral regurgitation. J Am Coll Cardiol 2009;54:860-5. [Crossref] [PubMed]
- Mirabel M, Iung B, Baron G, et al. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur Heart J 2007;28:1358-65. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017;135:e1159-95. [Crossref] [PubMed]
- De Backer O, Piazza N, Banai S, et al. Percutaneous transcatheter mitral valve replacement: an overview of devices in preclinical and early clinical evaluation. Circ Cardiovasc Interv 2014;7:400-9. [Crossref] [PubMed]
- Muller DWM, Farivar RS, Jansz P, et al. Transcatheter Mitral Valve Replacement for Patients With Symptomatic Mitral Regurgitation: A Global Feasibility Trial. J Am Coll Cardiol 2017;69:381-91. [Crossref] [PubMed]
- Perpetua EM, Reisman M. The Tendyne transcatheter mitral valve implantation system. EuroIntervention 2015;11 Suppl W:W78-9.
- Duncan A, Daqa A, Yeh J, et al. Transcatheter mitral valve replacement: long-term outcomes of first-in-man experience with an apically tethered device- a case series from a single centre. EuroIntervention 2017;13:e1047-57. [Crossref] [PubMed]
- Blanke P, Park JK, Grayburn P, et al. Left ventricular access point determination for a coaxial approach to the mitral annular landing zone in transcatheter mitral valve replacement. J Cardiovasc Comput Tomogr 2017;11:281-7. [Crossref] [PubMed]
- Murphy DJ, Ge Y, Don CW, et al. Use of Cardiac Computerized Tomography to Predict Neo-Left Ventricular Outflow Tract Obstruction Before Transcatheter Mitral Valve Replacement. J Am Heart Assoc 2017;6. [Crossref] [PubMed]
- Lutter G, Lozonschi L, Ebner A, et al. First-in-human off-pump transcatheter mitral valve replacement. JACC Cardiovasc Interv 2014;7:1077-8. [Crossref] [PubMed]
- Quarto C, Davies S, Duncan A, et al. Transcatheter Mitral Valve Implantation: 30-day Outcome of First-in-Man Experience With an Apically Tethered Device. Innovations (Phila) 2016;11:174-8. [Crossref] [PubMed]
- Bapat V, Rajagopal V, Meduri C, et al. Early Experience With New Transcatheter Mitral Valve Replacement. J Am Coll Cardiol 2018;71:12-21. [Crossref] [PubMed]
- Taramasso M, Maisano F. Transcatheter mitral valve interventions: pathophysiological considerations in choosing reconstruction versus transcatheter valve implantation. EuroIntervention 2015;11 Suppl W:W37-41.






