Characteristics and outcomes of patients with right-sided endocarditis undergoing cardiac surgery
Introduction
Right-sided infective endocarditis (RSIE) accounts for only 5–10% of all cases of infective endocarditis (IE) (1,2). However, an increase in the incidence of RSIE has recently been reported, primarily attributable to the global rise in the number of intravenous drug use (IVDU), along with an increased number of patients with cardiac implantable electronic devices (CIED) and a rising number of central venous catheters in clinical care (1,3-5). The vast majority of RSIE cases involve the tricuspid valve, with pulmonary valve involvement accounting for less than 10% of all right-sided cases (6).
The diagnosis of RSIE is frequently delayed, as right-sided murmurs often remain undetected and the usual presenting symptoms of persistent fever associated with pulmonary manifestations can be misdiagnosed as a respiratory tract infection (1,7). Consequently, the delay in timely diagnosis and initiation of appropriate antimicrobial therapy, leads to further complications, such as pulmonary infarcts, pulmonary abscesses, lung emphysema and rarely, pneumothorax (3,8).
Between 5–40% of patients with RSIE require surgical intervention (9-12), with reported operative mortalities between 0–15% for patients with isolated tricuspid valve IE (5,12,13). However, timing of surgery for RSIE is less clear than for left-sided infective endocarditis (LSIE). In RSIE, surgical treatment is mainly considered in patients with large vegetations, recurrent septic pulmonary emboli, persistent infection not responding to antibiotic therapy or worsening tricuspid regurgitation contributing to deteriorating right heart failure (5,10).
Given the low incidence of RSIE, available data of patients undergoing surgery for RSIE are scarce. Our aim was to analyze the clinical characteristics and predisposing risk factors of patients undergoing surgery for RSIE. A second objective was to investigate differences in the clinical presentation, microbiological findings and prognosis of surgical patients with RSIE compared to LSIE.
Methods
Study population
We retrospectively analyzed the clinical data of all 432 consecutive patients undergoing valve surgery for IE at our institution between January 2009 and December 2018. Acquired data included patients’ demographics and preoperative comorbidities, manifestation of IE according to the recently modified Duke Criteria (14) (echocardiographic and microbiological data), perioperative data [timing of operation, cardiopulmonary bypass time (CPB) and crossclamp time] and relevant clinical outcomes. Long-term follow-up was obtained by review of hospital records and interview of patients’ physicians. The follow-up time for survival was measured from the date of operation to either the date of death or the date of the last contact with the patient. The study protocol was approved by the institutional review board. Individual informed consent was waived due to the retrospective nature of the collected data.
Operative procedure
Cardiac surgery for IE was indicated according to the recent European Society of Cardiology (ESC) guidelines for the management of IE (15) and performed as previously described (16). The type of surgery was performed according to the surgeon’s preference and the extent of infection, with the aim of achieving radical debridement of infected tissue. Intraoperative transesophageal echocardiography was performed in all patients. Hemodynamic, catecholamine and blood transfusion management was administered at the discretion of the attending surgeon and anesthesiologist.
Statistical analysis
Unless otherwise indicated, continuous variables were described using mean values ± standard deviation (SD) or median [interquartile range (IQR)] according to the normality of their distribution and compared using unpaired t-test or Mann-Whitney U test as appropriate. Discrete variables were reported as percentages and tested by chi-squared test or, when validity conditions were not satisfied, by Fisher’s exact test. Missing data were not imputed and assumed to be missing at random. All reported P values are two-sided and considered statistically significant if ≤0.05. Statistical analyses were performed using SPSS Statistics Version 25 (IBM Corp., Armonk, NY, USA)
Results
Preoperative characteristics
The preoperative clinical profile of our surgical IE population is summarized in Table 1. A total of 403 patients (93.3%) underwent surgery for LSIE and twenty-nine patients (6.7%) for RSIE. Patients undergoing surgery for RSIE were significantly younger [47.5 (40.4–69.3) vs. 65.1 (53.7–74.6); P=0.008] and presented with less comorbidities, such as hypertension (41.4% vs. 65.3%; P=0.010) and coronary artery disease (6.9% vs. 29.0%; P=0.010). Although not statistically significant, there was a trend towards less diabetes mellitus (13.8% vs. 27.8%; P=0.100), peripheral artery disease (3.4% vs. 8.2%; P=0.310) and preoperative stroke (6.9% vs. 13.2%; P=0.293) in RSIE patients. Conversely, smoking was more prevalent in RSIE (37.9% vs. 21.3%; P=0.039). EuroSCORE II was lower in RSIE [6.0 (4.0–9.5)] compared to LSIE [8.0 (5.0–11.0); P=0.019].
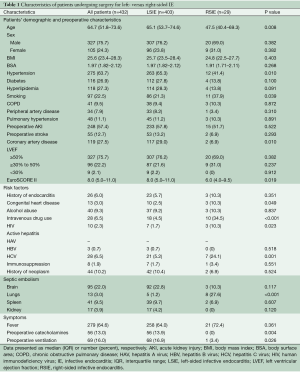
Full table
In contrast to the lower rate of comorbidities, patients with RSIE presented with more risk factors for IE. Hence, higher rates of IVDU (34.5% vs. 4.5%; P<0.001), HIV (10.3% vs. 1.7%; P=0.023) and HCV infection (24.1% vs. 5.2%; P=0.001) were more frequently diagnosed in RSIE. In addition, congenital heart disease was more prevalent in RSIE compared to LSIE (10.3% vs. 2.5%; P=0.049).
RSIE patients suffered from more septic pulmonary emboli (27.6% vs. 1.2%; P<0.001), whereas LSIE patients presented with more severe manifestation of disease, reflected by greater preoperative catecholamine requirements (0% vs. 13.9%; P=0.004) and preoperative ventilation (3.4% vs. 16.9%; P=0.026).
IE manifestation
A total of 403 patients underwent surgery for LSIE. Only twenty-nine patients in the cohort were diagnosed with RSIE, including twenty-seven cases of tricuspid valve infection (93.1%) and two cases of pulmonary valve infection (6.9%). In the RSIE group, eleven patients (37.9%) showed concomitant left-sided infection: six patients were diagnosed with concomitant aortic involvement and four patients with concomitant mitral valve involvement. One patient was operated due to RSIE with concomitant aortic and mitral valve IE.
Preoperative echocardiography revealed significantly larger vegetations in patients with RSIE compared to LSIE [2.2 (1.8–2.9) vs. 1.5 (1.0–2.0) cm; P=0.007]. Perivalvular infection was comparable in both groups (Table 2).
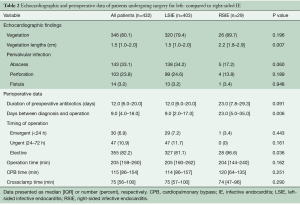
Full table
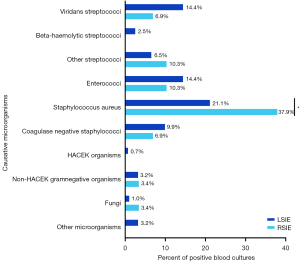
Microbiological findings are depicted in Figure 1. The three most detected causative microorganisms were Staphylococcus aureus, Streptococcus spp. and Enterococcus spp. The proportion of Staphylococcus aureus IE was significantly higher in RSIE compared to LSIE (37.9% vs. 21.1%; P=0.035).
With regard to the timing of operation, cardiac surgery for RSIE was performed later than in LSIE. Hence, the time between diagnosis and operation was significantly longer [23.0 (5.0–35.0) vs. 9.0 (2.0–17.0) days; P=0.006] and the proportion of elective surgery higher (96.6% vs. 81.1%; P=0.036). There was no difference in the duration of operation, reflected in similar operation, cardiopulmonary bypass (CPB) and crossclamp times (Table 2).
Postoperative clinical outcomes
Postoperative clinical outcomes are summarized in Table 3. There was no statistical difference in 30-day mortality between RSIE and LSIE (6.9% vs. 14.6%, P=0.373), whereas 1-year mortality was lower in RSIE compared to LSIE (6.9% vs. 26.1%; P=0.045). The incidence of postoperative complications, such as re-exploration for bleeding, postoperative stroke, postoperative hemodialysis and tracheostomy did not differ between groups.
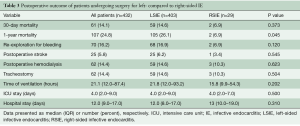
Full table
Subgroups of patients with RSIE
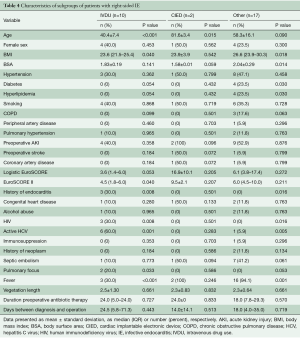
Characteristics of the subgroups of patients with RSIE are depicted in Table 4. IVDU was present as a risk factor in ten RSIE patients (34.5%). These patients were significantly younger (40.4±7.4; P<0.001) and had a lower body mass index (BMI) [23.6 (21.5–25.4); P=0.040]. IVDU patients presented with more HIV (30% vs. 0%; P=0.008) and HCV (60% vs. 5.3%; P=0.001) infection.
Full table
Only two patients (6.9%) showed a CIED-related endocarditis. They were significantly older with a mean age of 81.6±3.4 (P=0.015). However, due to the small number of patients with CIED, we could not perform a meaningful subgroup analysis of patients with CIED-related RSIE.
Patients without IVDU or CIED presented with more comorbidities, such as diabetes mellitus (23.5% vs. 0%; P=0.030) and hyperlipidemia (23.5% vs. 0%; P=0.030). Contrarily to patients with IVDU, most other patients presented with fever (94.1% vs. 41.7%; P=0.001).
Discussion
The incidence of RSIE compared to LSIE has been reported to be relatively low (1,6). In our IE cohort, only 6.7% underwent cardiac surgery for RSIE. The tricuspid valve was affected in the majority of RSIE (93.1%), with only two cases (6.9%) of pulmonary valve IE. Compared with LSIE, RSIE is usually associated with a favorable prognosis and low mortality rates (3,17). We observed a 30-day mortality of 6.9% in patients undergoing surgery for RSIE, consistent with previous reports, showing mortality rates ranging from 5% to 12.5% (18,19). However, we could not detect a difference in 30-day mortality. This might be attributed to a high proportion (37.9%) of concomitant LSIE involvement in our RSIE group.
Diagnosis of RSIE is often delayed, since respiratory, rather than systemic signs of IE predominate (1). A variety of complications caused by septic pulmonary emboli have been described, such as pulmonary infarction, pulmonary abscesses, bilateral pneumothoraces, pleural effusions and empyema (9). In line with this, we observed a significantly higher rate of septic pulmonary emboli in patients undergoing surgery for RSIE compared to LSIE (27.6% vs. 1.2%; P<0.001).
The RSIE population can be distinguished by patients with IVDU, implanted cardiac electronic devices and patients with central venous lines or dialysis access (3). IVDU has been described as a leading predisposing factor and accounts for an increasing incidence in developed countries (1,20). There have been reports that 30–40% of all RSIE cases are associated with IVDU (5,21). This is in line with our findings, showing a proportion of 34.5% with IVDU in the RSIE cohort. In contrast, RSIE due to CIED accounts for only 9% in the literature (2,22). Similarly, we found a rate of 6.9% of CIED-related RSIE.
IVDU patients are more likely to be younger, and their high likelihood of continued drug use is a serious clinical challenge (5). In line with this, we observed that patients in the subgroup with IVDU were significantly younger (40.4±7.4; P<0.001) and presented with high rates of HIV (30%) and HCV infection (60%).
Several risk factors for Staphylococcus aureus infection in RSIE have been reported, such as higher rates of nasal and cutaneous Staphylococcus aureus colonization and contaminated drug use (3,23,24). We found Staphylococcus aureus was the most common causative microorganism in the IE cohort, with a significantly higher rate of Staphylococcus aureus infection in RSIE compared to LSIE (37.9% vs. 21.1%; P=0.035). These findings are consistent with a previous study by Pfannmueller et al. reporting a 43% incidence of Staphylococcus aureus infection, followed by coagulase-negative staphylococci and Enterococcus faecalis (19). Several risk factors for mortality in patients with RSIE have been described, including Staphylococcus aureus infection (5,6,15), fungal etiology or large vegetations (25,26). The presence of large vegetations >2 cm has been correlated with an increased risk of short- and long-term complications and has been associated with a higher mortality (1,9,25,27). Akinosoglou et al. hypothesized that antibiotic penetration is impaired in large vegetations, complicating the eradication of more resistant organisms (1). We found significantly longer vegetations in patients undergoing surgery for RSIE compared to LSIE.
With increasing experience in cardiac surgery for RSIE, lower or similar in-hospital mortality rates have been described compared to antibiotic therapy alone (5,28). Currently, cardiac surgery for RSIE is suggested in heart failure due to tricuspid valve regurgitation with poor response to medical therapy, tricuspid valve vegetations larger than 2 cm and recurrent emboli despite antimicrobial therapy (15,29,30). Principles of surgery for RSIE include radical debridement of vegetations and infected tissue (10) and valve repair whenever possible (5). Especially in IVDU, valve repair has the advantage of avoiding or minimizing the implantation of foreign material while preserving the function of the valve (9). If the valve is largely destroyed and a replacement is necessary, a bioprosthesis is preferable to a mechanical valve, which requires long-term anticoagulation in patients in whom IVDU is predominant and non-compliance is a major issue (5,10,13,28). Therefore, we suggest that earlier cardiac surgery might prevent further destruction of the leaflets, improve the likelihood of a good repair and decrease RSIE-related mortality by reducing the risk of right heart failure.
Our study has several limitations that need to be considered for the interpretation of the results. First, this is a single-center study with retrospectively collected data for a limited number of patients. Second, although the sample size of our IE cohort appears to be adequate for a single institution IE collective, the number of patients with RSIE is very small due to the low incidence of RSIE. Hence, we could not perform a powerful univariate and multivariable analysis for risk stratification.
In summary, our data indicate that surgery for RSIE can be performed with low operative mortality. However, larger multicenter prospective trials are needed in order to provide more reliable data on the clinical profile and postoperative course of patients undergoing surgery for RSIE.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Akinosoglou K, Apostolakis E, Marangos M, et al. Native valve right sided infective endocarditis. Eur J Intern Med 2013;24:510-9. [Crossref] [PubMed]
- Naidoo DP. Right-sided endocarditis in the non-drug addict. Postgrad Med J 1993;69:615-20. [Crossref] [PubMed]
- Chahoud J, Sharif Yakan A, Saad H, et al. Right-Sided Infective Endocarditis and Pulmonary Infiltrates: An Update. Cardiol Rev 2016;24:230-7. [Crossref] [PubMed]
- Cabell CH, Jollis JG, Peterson GE, et al. Changing patient characteristics and the effect on mortality in endocarditis. Arch Intern Med 2002;162:90-4. [Crossref] [PubMed]
- Hussain ST, Witten J, Shrestha NK, et al. Tricuspid valve endocarditis. Ann Cardiothorac Surg 2017;6:255-61. [Crossref] [PubMed]
- Murdoch DR, Corey GR, Hoen B, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med 2009;169:463-73. [Crossref] [PubMed]
- Fernandez Guerrero ML, Alvarez B, Manzarbeitia F, et al. Infective endocarditis at autopsy: a review of pathologic manifestations and clinical correlates. Medicine (Baltimore) 2012;91:152-64. [Crossref] [PubMed]
- Alexiou C, Langley SM, Stafford H, et al. Surgery for active culture-positive endocarditis: determinants of early and late outcome. Ann Thorac Surg 2000;69:1448-54. [Crossref] [PubMed]
- Moss R, Munt B. Injection drug use and right sided endocarditis. Heart 2003;89:577-81. [Crossref] [PubMed]
- Akinosoglou K, Apostolakis E, Koutsogiannis N, et al. Right-sided infective endocarditis: surgical management. Eur J Cardiothorac Surg 2012;42:470-9. [Crossref] [PubMed]
- Ortiz C, Lopez J, Garcia H, et al. Clinical classification and prognosis of isolated right-sided infective endocarditis. Medicine (Baltimore) 2014;93:e137. [Crossref] [PubMed]
- Musci M, Siniawski H, Pasic M, et al. Surgical treatment of right-sided active infective endocarditis with or without involvement of the left heart: 20-year single center experience. Eur J Cardiothorac Surg 2007;32:118-25. [Crossref] [PubMed]
- Gaca JG, Sheng S, Daneshmand M, et al. Current outcomes for tricuspid valve infective endocarditis surgery in North America. Ann Thorac Surg 2013;96:1374-81. [Crossref] [PubMed]
- Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000;30:633-8. [Crossref] [PubMed]
- Authors/Task Force M, Habib G, Lancellotti P, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC)Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015;36:3075-128. [Crossref] [PubMed]
- Weber C, Gassa A, Rokohl A, et al. Severity of presentation, not sex, increases risk of surgery for infective endocarditis. Ann Thorac Surg 2019;107:1111-7. [Crossref] [PubMed]
- Fernandez Guerrero ML, Gonzalez Lopez JJ, Goyenechea A, et al. Endocarditis caused by Staphylococcus aureus: A reappraisal of the epidemiologic, clinical, and pathologic manifestations with analysis of factors determining outcome. Medicine (Baltimore) 2009;88:1-22. [Crossref] [PubMed]
- Hilbig A, Cheng A. Infective Endocarditis in the Intravenous Drug Use Population at a Tertiary Hospital in Melbourne, Australia. Heart Lung Circ 2019. [Epub ahead of print].
- Pfannmueller B, Kahmann M, Davierwala P, et al. Tricuspid Valve Surgery in Patients with Isolated Tricuspid Valve Endocarditis: Analysis of Perioperative Parameters and Long-Term Outcomes. Thorac Cardiovasc Surg 2017;65:626-33. [Crossref] [PubMed]
- Chambers HF, Morris DL, Tauber MG, et al. Cocaine use and the risk for endocarditis in intravenous drug users. Ann Intern Med 1987;106:833-6. [Crossref] [PubMed]
- Baraki H, Saito S, Al Ahmad A, et al. Surgical treatment for isolated tricuspid valve endocarditis- long-term follow-up at a single institution. Circ J 2013;77:2032-7. [Crossref] [PubMed]
- Revilla A, Lopez J, Villacorta E, et al. Isolated right-sided valvular endocarditis in non-intravenous drug users. Rev Esp Cardiol 2008;61:1253-9. [Crossref] [PubMed]
- Tuazon CU, Sheagren JN. Increased rate of carriage of Staphylococcus aureus among narcotic addicts. J Infect Dis 1974;129:725-7. [Crossref] [PubMed]
- Ortiz-Bautista C, Lopez J, Garcia-Granja PE, et al. Current profile of infective endocarditis in intravenous drug users: The prognostic relevance of the valves involved. Int J Cardiol 2015;187:472-4. [Crossref] [PubMed]
- Hecht SR, Berger M. Right-sided endocarditis in intravenous drug users. Prognostic features in 102 episodes. Ann Intern Med 1992;117:560-6. [Crossref] [PubMed]
- Martin-Davila P, Navas E, Fortun J, et al. Analysis of mortality and risk factors associated with native valve endocarditis in drug users: the importance of vegetation size. Am Heart J 2005;150:1099-106. [Crossref] [PubMed]
- Okonta KE, Adamu YB. What size of vegetation is an indication for surgery in endocarditis? Interact Cardiovasc Thorac Surg 2012;15:1052-6. [Crossref] [PubMed]
- Dawood MY, Cheema FH, Ghoreishi M, et al. Contemporary outcomes of operations for tricuspid valve infective endocarditis. Ann Thorac Surg 2015;99:539-46. [Crossref] [PubMed]
- Baddour LM, Wilson WR, Bayer AS, et al. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation 2015;132:1435-86. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:2440-92. [Crossref] [PubMed]






