Contemporary approaches in the use of extracorporeal membrane oxygenation to support patients waiting for lung transplantation
Introduction
Extracorporeal membrane oxygenation (ECMO) is a powerful support modality capable of sustaining patients with respiratory or circulatory failure when skillfully deployed (1). Successful use of ECMO to support patients previously considered unsalvageable with conventional methods has prompted an exponential increase in the number of ECMO centers and its expanding application as an experimental therapy for acute respiratory distress syndrome (ARDS) (2). Parallel to its adoption as a bridge to recovery for acute respiratory failure, transplant registries report increasing use of ECMO as a bridge to transplant for critically-ill patients.
Lung transplantation and the lung allocation score (LAS) era
Despite recent medical advances (3,4), lung transplantation remains the only potentially curative therapy for end-stage lung disease. Adoption of the LAS in 2005 heralded a significant shift in the care of patients with advanced pulmonary disease listed for lung transplantation (5). The previous system awarded organs based on time on the waiting list. This approach incentivized listing of healthier patients capable of surviving longer waiting periods to increase probability of receiving organs. Implementation of the new LAS system prioritizes patients with lower projected transplant-free survival to increase donor organ allotment to critically ill patients.
Prioritization of sicker patients coincided with doubling of mortality rates for patients on the waiting list from 8.6 to 16.5 per 100 waitlist-years from 2005 to 2015, respectively (6). Although the new allocation system achieved the goal of decreasing waiting list time for patients with the most urgent need for transplant, it motivated a change in the clinical care of critically-ill patients with end-stage lung disease. Transplant centers increasingly confronted two intertwined challenges: (I) how to optimally maintain the vitality of patients with advanced disease to improve survival through the rigors of surgery and post-operative recovery and (II) how to extend the lives of critically-ill patients at risk of imminent death while on the cusp of a life-saving transplant.
ECMO as bridge to transplant
ECMO was initially considered a contraindication to transplant, given reported one-year survival rates of 44% for pre-transplant patients maintained on extracorporeal support (7). Pre-transplant mechanical ventilation was also known to be an independent risk factor for increased post-transplant mortality (8). The combination of technological advancements with improved understanding of device use, more widespread availability and limitations of conventional support led transplant centers to re-evaluate use of ECMO as a pre-transplant support modality (9,10).
Informed by single-center reports of successful use, transplant centers are rapidly embracing ECMO as a means to maintain patient viability and survival to bridge-to-transplant. One center reported a six-month survival rate of 80% in patients supported on ECMO pre-transplant, compared to 50% of patients maintained on mechanical ventilation (11). Another experienced center reported survival rates of 84% at two years post-transplant for patients supported with ECMO prior to surgery (12). A retrospective analysis demonstrated the importance of patient volume and ECMO expertise as high-volume centers reported one-year survival rates of 80.8% for patients bridged to transplant by ECMO compared to only 61.9% for low volume centers (13). Despite increased use, questions persist regarding: (I) how to optimally support patients waiting for lung transplant; (II) how support options are determined by the underlying disease process; and (III) when to initiate support. This article presents the use of ECMO as a bridging strategy to lung transplantation and details its uses, current outcomes, challenges and future opportunities for development.
Overview of ECMO
Historical perspective
ECMO is an adaptation of the cardiopulmonary bypass circuit modified for prolonged use in the intensive care unit (14). Following initial case reports of success in rescuing patients with respiratory failure (15), subsequent clinical trials comparing ECMO to contemporary critical care approaches for respiratory failure failed to demonstrate benefit (16,17). While these early failures impeded development of the technology, ECMO succeeded in becoming the established care modality for neonates with cyanotic heart disease and was also shown to improve outcomes in severe cases of respiratory failure from meconium aspiration (18). From this foothold in quaternary pediatric hospitals, ECMO gradually expanded to become a familiar, if uncommon, component of cardiac surgical intensive care units employed to maintain postcardiotomy patients unable to wean from cardiopulmonary bypass.
The H1N1 influenza pandemic in 2009 led to an exponential increase in ECMO use for patients with respiratory failure (19). The pandemic affected primarily younger patients commonly manifesting with severe disease (20). This surge in previously healthy young patients with influenza pneumonia and ARDS spurred a renewed interest in the use of ECMO support in adults. The CESAR trial, published during the H1N1 pandemic, demonstrated improved outcomes in ARDS patients cared for at ECMO centers (21). In the decade following the pandemic, the number of ECMO centers and patients supported on ECMO has grown dramatically (22). Renewed interest in this technology and its increased availability provided transplant centers with the opportunity to trial ECMO as a means of supporting critically ill pre-transplant patients.
Modern ECMO circuit components
The modern ECMO circuit used for adult patients consists of the following core components: (I) mechanical pump; (II) gas exchange device; and (III) vascular cannula and circuit tubing (23). Blood is drawn up by the pump and passed through the gas exchange device, termed an oxygenator, to return oxygenated blood to the patient. Centrifugal pumps have largely replaced the peristaltic pumps of early circuits due to decreased hemolysis and reduced activation of leukocytes and platelets. Modern oxygenators are manufactured from bundles of hollow fibers (24). Sweep gas flows through the fibers while blood passes around fiber bundles to achieve gas exchange. The advent of polymethylene to make the fiber bundles has increased oxygenator durability from days to weeks with limited plasma leakage into the hollow fibers that acts to degrade device performance. Vascular cannulas draw venous blood and return it to either the venous or arterial circulation depending on the specific cannulation strategy.
Cannula placement determines the therapeutic support modality provided by ECMO. Terminology is not standardized and is variably defined in the literature. For this article, ECMO is classified as follows:
- VV ECMO—venovenous ECMO: blood is drawn from the systemic venous circulation, passed through the circuit, and returned to the systemic venous circulation;
- VA ECMO—venoarterial ECMO: blood is drawn from the systemic venous circulation, passed through the circuit, and returned to the systemic arterial circulation;
- VAV ECMO—venoarterial-venous ECMO: blood is drawn from the systemic venous circulation, passed through the circuit, and then a fraction of the circuit flow is returned to the systemic arterial circulation while the remaining fraction return to the systemic venous circulation.
This terminology covers most commonly employed configurations in clinical practice. VV ECMO achieves gas exchange to provide support for lung failure while requiring adequate heart function to maintain systemic perfusion. VA ECMO shunts blood from the systemic venous to the systemic arterial circulation to provide perfusion support. VA ECMO profoundly alters systemic hemodynamics with the specific effects a function of how support is titrated and the patient’s physiological state. VAV ECMO is a less commonly used strategy for patients with combined respiratory and circulatory failure and will not be further discussed.
Operation of the ECMO circuit relies on modulation of only a few parameters to adjust support: pump speed, sweep gas flow rate and sweep gas composition. The flow through the ECMO circuit is a function of: (I) pump speed; (II) afterload to the pump including circuit resistance and the patient’s vascular resistance; (III) patient’s volume status and the negative pressure at which there is vascular collapse around the withdrawal cannula; and (IV) cardiac function. Support provided by ECMO is a complex integration of device operation and patient physiological state and anatomy.
ECMO circuit complications
The ECMO circuit constitutes an external circulation exposing blood to non-biological surfaces. Blood contact with the circuit tubing and mechanical forces generated by the pump and passage through the oxygenator activates platelets, leukocytes, the coagulation and fibrinolytic cascades and complement (25). Platelet and coagulation cascade activation promote clot formation in the oxygenator and induce the risk of thromboembolism. Advances in biomaterials have led to the development of circuit components with surface-bonded anticoagulants that may reduce the risk of clot formation (26). Despite these improvements, anticoagulation remains common practice for patients on ECMO to reduce thromboembolic risk and extend oxygenator lifespan. Although this is common practice, the optimal anticoagulation strategy is unknown (27). The risk of clot formation must also be balanced with the risk of bleeding as platelet activation reduces platelet functionality and mechanical destruction by the pump may create a functional von Willebrand factor deficiency.
Hemolysis, caused by the pump or through shear stress induced by high pressure gradients across the vascular cannula, is a common complication that limits the amount of support provided by the ECMO circuit (28). Consumption of blood cell components may require transfusions during the pre-transplant period to maintain acceptable red blood cell and platelet levels, risking antibody formation against potential donor antigens. Further effects of hemolysis—renal injury, inflammation, elevated bilirubin levels—may complicate evaluation of a patient’s ongoing suitability for transplant.
The presence of a large bore percutaneous cannula is a significant risk factor for infection (29). Unlike other vascular catheters, which are easier to care for and can more readily be replaced, ECMO cannulas provide active life support and are not amenable to easy substitution. Akin to a drive line infection in a ventricular assist device (VAD) patient, cannula-associated infections require ongoing antibiotic treatment until the cannula is removed. In the transplant patient, evidence of active infection can prompt temporary deactivation to determine the source of infection and clinical stability prior to reactivation.
Clinical challenges in managing the pre-lung transplant patient
The shortage of suitable donor lungs limits organ transplantation as a cure for patients with advanced pulmonary disease. The lack of available organs underscores the entire evaluative process for prospective transplant candidates, as transplant centers strive to ensure scarce donor organs are allocated to patients capable of realizing meaningful benefit. The screening steps candidates undergo can be grouped into the following categories (30):
- Disease diagnosis and severity to determine allocation priority;
- Functional and nutritional status to assess ability to withstand rigors of transplant surgery;
- Evaluation for other disease processes that might prematurely shorten life expectancy or be critically exacerbated by the transplant process and post-transplant care;
- Psychosocial factors that impair ability to maintain reliable care to maximize benefit of the allograft.
The evaluative process is extensive and often time-consuming, placing patients at risk for clinical decline prior to completion of screening and activation of candidacy. The potential for decline is an important consideration in the pre-transplant planning process for each individual candidate.
Clinical deterioration is dependent on the underlying disease, which in turn guides the decision whether to deploy ECMO or conventional support strategies. Chronic obstructive lung disease (COPD), idiopathic pulmonary fibrosis (IPF) and cystic fibrosis (CF) account for more than 75% of the indications for all lung transplant recipients (31). Familiarity with the physiological manifestations of end-stage disease for each of these conditions guides the appropriate cannulation and extracorporeal support strategy (Table 1). Pulmonary arterial hypertension, although accounting for only approximately 3% of all lung transplant indications, deserves special consideration given its unique physiology and how it can inform management approaches applicable to subgroups of other primary lung diseases complicated by right ventricular (RV) dysfunction.
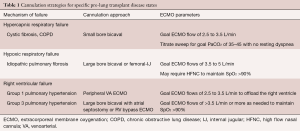
Full table
Chronic obstructive pulmonary disease
COPD is the most common indication worldwide for lung transplantation (32). The natural course of COPD is often protracted and places patients at risk of severe deconditioning prior to transplant with patients experiencing a variety of physiological derangements secondary to disease progression (33). Both hypo- and hypercapnia are associated with increased mortality in COPD patients with hypocapnia hypothesized to lead to respiratory muscle fatigue and subsequent failure (34). Patients often experience pulmonary cachexia with hypercapnia associated with deterioration of muscle function (35). Pulmonary hypertension (PH) is prevalent in advanced disease and is associated with increased mortality (36). The variability in physiological manifestations in advanced COPD complicates determination of the appropriate support strategy with the approach tailored to offset the dominant impairment.
Interstitial pulmonary fibrosis
Interstitial lung diseases (ILDs), and specifically IPF, typically manifest as restrictive patterns in spirometry with decreased gas diffusion capacity (37). Despite new anti-fibrotic treatments, life expectancy following diagnosis with IPF is dismal with lung transplantation being the only proven therapy to provide a survival benefit (38). Patients with IPF experience progressive fibrosis and worsening impairment in gas exchange, manifesting as hypoxia and decreased exercise capacity (39). During acute exacerbations or in late stages of the disease, patients are typically profoundly hypoxic and frequently require high levels of supplemental oxygen to maintain normoxia (40). Use of invasive mechanical ventilation is complicated by decreased lung compliance and its initiation can cause respiratory collapse due to the loss of respiratory drive by induction of anesthesia. PH is common in advanced disease and may affect an estimated 30% to 50% of patients (41).
Cystic fibrosis
CF is a disease caused by mutations in the CF transmembrane conductance regulator gene (CFTR) that results in pulmonary disease, pancreatic insufficiency and sinus disease, among other manifestations of variable penetrance (42). Patients experience thick, tenacious secretions, chronic productive cough, and recurrent bacterial airway infections. Pulmonary disease is the leading cause of mortality in CF and is characterized by obstructive airway disease, bronchiectasis and recurrent exacerbations that increase with frequency and severity as the disease progresses. Massive hemoptysis is an additional complication that occurs in approximately 4% of patients with an annual average incidence of 1% (43).
Pulmonary arterial hypertension
PH is classified by etiology into five groups by the World Health Organization (44). Group 1 PH consists of both idiopathic and heritable forms of the disease which are both characterized by pulmonary arterial hyperplasia and hypertrophy. These vascular changes lead to elevated pulmonary arterial pressures that eventually cause decreased cardiac output and right heart failure and lead to death unless lung transplantation is performed.
Conventional support and the motivation for extracorporeal support
High flow nasal cannula (HFNC), capable of delivering humified air at flows up to 60 L/min, and mechanical ventilation are the mainstays of conventional respiratory support for patients with end-stage lung disease. For critically-ill, non-transplant candidates, conventional therapy is relied on to provide homeostasis to permit recovery in the setting of a treatable exacerbation, or to maintain the patient to allow time for evaluating goals of care. For the pre-transplant patient, mere existence is not sufficient as the means of support must both sustain the patient’s survival and maintain or even improve the patient’s functional status in preparation for transplant.
All forms of conventional therapy for respiratory failure share a common limitation in their reliance on diseased organs to achieve physiological goals. Whether through increased delivery of oxygen to alveoli or application of positive pressure to augment ventilation, conventional therapy uses the lungs to accomplish gas exchange. This principle limits utility of conventional therapy in advanced disease when further functional augmentation fails to obtain adequate gas transfer. Conventional therapy may even hasten the progression of underlying disease through hyperoxia or barotrauma (45).
Despite mechanistic limitations of conventional therapies, they are well understood and easily accessible. For IPF patients suffering from hypoxic respiratory failure, HFNC is a non-invasive approach to relieve hypoxia and permit physical activity. Patients in whom HFNC is insufficient may benefit from invasive mechanical ventilation through recruitment of atelectatic lung and improved ventilation-perfusion matching (46). However, mechanical ventilation in IPF patients is not without risk. Alteration of respiratory mechanics with anesthesia induction and during the transition to positive pressure may provoke catastrophic failure, while poor lung compliance limits traditional ventilator strategies (47,48). Similar challenges confront management of CF exacerbations in patients with advanced disease (49). Recurrent infections and inflammation reduce lung compliance and impair ventilation resulting in hypercapnic respiratory failure. In severe cases, mechanical ventilation is unable to provide adequate support prompting the need for extracorporeal approaches to achieve gas exchange.
Criteria to initiate ECMO support
Each center balances its reliance on the traditional support technologies against efforts to implement new methods of maintaining critically ill pre-transplant patients. The decision to pursue extracorporeal support measures the benefit of providing clinical stability and the risk of pursuing an invasive therapy in a fragile patient population. The transplant center’s capability and experience are important considerations for determining the specific approach for a given patient.
There is currently no definitive evidence to guide when to initiate extracorporeal support. At our center, in an effort to promote consultation before a patient becomes critically ill, we recommend consideration of ECMO support if any of the following conditions are met:
- Inability to maintain SpO2 >90% with HFNC at rest;
- Inability to participate in physical therapy with any form of supplemental oxygen therapy;
- Evidence of hemodynamic instability due to impaired gas exchange;
- Need for positive pressure ventilation to maintain gas exchange;
- Exertional chest pain in the setting of RV failure;
- End organ impairment due to RV failure or the need for inotropic support.
Multiple factors are considered when initiating extracorporeal support for bridge to lung transplantation: (I) physiological requirements of support; (II) cannulation strategy; (III) available skilled personnel and resources; (IV) patient stability and urgency to initiate support. The ECMO physician balances these considerations at the time of cannulation to determine the optimal available course. Worsening end-stage lung disease typically presents over a manner of days to weeks and provides transplant centers with opportunities to prepare for the initiation of ECMO support in the event patients are not adequately supported by traditional therapies. Exceptions to this exist, notably in the setting of rapidly progressive exacerbations of ILD or CF, which may require emergent initiation of ECMO support.
Approach to veno-venous ECMO support in the pre-lung transplant patient
Patients with end-stage lung disease manifesting as hypoxic, hypercapnic, or mixed respiratory failure are candidates for VV ECMO. Deployed in this configuration, ECMO removes deoxygenated blood and returns oxygenated blood to the systemic venous circulation (Figure 1). This cannulation approach provides partial lung support while relying on intact cardiac function to maintain end organ perfusion. Ventilation-perfusion matching in the lung may be altered through the effects of increased oxygen content in the pulmonary arteries, which may also lower pulmonary vascular resistance from reduced hypoxic vasoconstriction.
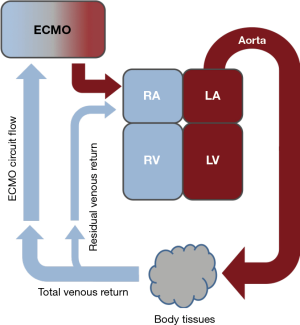
Our center relies on two primary cannulation strategies to support patients waiting for lung transplantation: (I) dual lumen bicaval cannulation via the right internal jugular (IJ) vein and (II) femoral vein-right IJ vein cannulation. Our preference is to perform cannulations in the operating room environment when possible given the larger physical space, access to additional imaging and surgical equipment if needed and ease for additional personnel to be present for support and training purposes. For urgent cannulations performed at the bedside, we rely on surface ultrasound to obtain vascular access and guide cannula placement. Multiple reviews of cannulation techniques and practices are present in the literature (50-52). Our approach is presented below as a comparison and to detail techniques tailored to the pre-lung transplant population.
Dual lumen bicaval cannulation
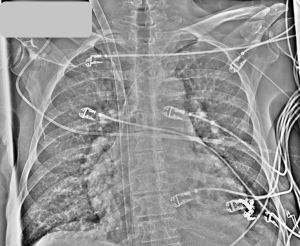
Our method for insertion of a dual lumen bicaval cannula (Figure 2) is to employ a multistep process to reduce risk of potentially catastrophic patient injury and to increase likelihood of optimal placement. We typically use surface echo given its ease of use and ability to visualize each step of the process. Assuming sterile technique and full barrier precautions, the key steps consist of the following:
- Place central venous catheter (CVC) capable of accommodating a 0.035" guidewire in the right IJ vein under ultrasound guidance. The catheter insertion point is more proximal than standard central venous catheter (CVC) placement to accommodate the large cannula diameter of the ECMO cannula. We recommend this step to allow for stable access of the vessel to facilitate wire manipulation;
- Advance guidewire into the inferior vena cava (IVC) under ultrasound visualization. We recommend using a stiffer guidewire, such as the Amplatz extra stiff 160-cm wire, to reduce likelihood of kinking during dilation. The stronger wire reduces risk of cannula redirection into the RV during advancement which may cause perforation of the ventricle. We use ultrasound guidance throughout wire advancement to avoid potential tricuspid valve injury and to guide the wire tip into the IVC distal to the hepatic vein;
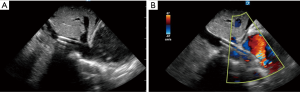
- We serially dilate using a combination of progressively larger dilators and blunt dissection as needed to allow for cannula advancement. We insert the cannula under ultrasound guidance (Figure 3A) until the external bifurcation is at the insertion point in the skin prior to removal of the internal dilator. We recommend this as the internal dilator extends significantly beyond the cannula tip and we do not advise advancing the cannula without dilator and guidewire in place;
- For intubated patients, we pause ventilation after inspiration during dilation and cannula advancement to reduce the likelihood of air embolism through the skin tract and into the jugular vein;
- Prior to cannula to circuit connection, we add additional tubing to facilitate ambulation and patient movement. This practice modestly increases circuit volume and the dilutional effect of initiating ECMO support;
- Cannula positioning is determined by a combination of surface ultrasound imaging of flow towards the tricuspid valve (Figure 3B) and physiological data optimizing oxygen saturation and hemodynamics while minimizing blood recirculation in the circuit;
- The cannula is placed with the patient lying supine with the head directed anteriorly. In the event of decreased flow or issues with cannula position, we advise return of the patient to this position until an expert provider can further evaluate.
Femoral-IJ cannulation
Femoral-IJ cannulation is less complicated with lower risk of patient injury and reduced requirements for advanced skills in bedside imaging to guide and optimize cannula placement. We typically use a 25-Fr 60-cm long multistage drainage cannula for blood withdrawal and a 17- or 19-Fr 18-cm long cannula for return of oxygenated blood into the right IJ artery. Our preference is to use this cannulation strategy for patients with profound hypoxic respiratory failure or if the patient will require higher circuit blood flow given the large diameter of the withdrawal cannula. We also use this approach for urgent bedside cannulations when there is inadequate time to prepare resources for safe bicaval cannulation.
Awake cannulation
The ability of ECMO to maintain a patient with end-stage lung disease with minimal additional support motivates cannulation when non-invasive therapies are inadequate (10,53,54). The clinical challenge with transitioning to this approach is to safely and reliably cannulate the awake patient in respiratory distress. Standard VV ECMO cannulation, and the method detailed here, involves accessing the right IJ vein and dilating the vessel to accommodate cannula ranging from 15 to 31 Fr in diameter. Spontaneously breathing patients are at significant risk of air entrainment into the venous circulation and potentially catastrophic air embolism to the RV. In our experience, this problem is particularly acute in IPF patients accustomed to the need to generate significant respiratory effort during inspiration. Patients with advanced lung disease are also commonly hypovolemic in the setting of aggressive efforts at diuresis to improve lung function. This relative hypovolemia impedes the ability to gain vascular access and places patients at risk of air entrainment during dilator removal due to the lack of venous blood return to adequately backfill the cannula. We recommend the presence of two skilled operators for awake cannulations to provide support and reduce the risk of potentially catastrophic error.
We use modest doses of short-acting narcotics and an infusion of dexmedetomidine to provide anxiolysis during cannulation while not impairing respiratory drive or altering hemodynamics. We rely on local anesthetic to maintain patient comfort and dedicate a provider to engage with the patient throughout the procedure to reduce patient anxiety. Successful awake cannulation avoids the need for intubation. This is advantageous in patients with right heart failure at risk for hemodynamic collapse during the transition from negative to positive pressure ventilation.
Hypoxic respiratory failure
An estimated 97% of oxygen in the blood is transported bound to hemoglobin with the remainder present as dissolved gas. The efficacy of VV ECMO in supporting patients with hypoxic respiratory failure is a function of the fraction of venous return and thereby the fraction of circulating hemoglobin, diverted to the extracorporeal circuit prior to return to the venous circulation. As the VV configuration is not able to entrain all of the venous return, VV ECMO in hypoxic respiratory failure functions as partial lung support. Typical ECMO flow rates are 3–4 L/min using bicaval dual lumen cannula and 3.5 to 5 L/min using femoral-IJ cannulation depending on the factors listed previously.
For ARDS patients with hypoxic respiratory failure maintained on ECMO, one aspect of clinical management is to reduce cardiac output to ensure adequate entrainment of venous return into the extracorporeal circuit. Strategies include volume resuscitation, use of beta-blockers to slow heart rate and transfusion to increase oxygen-carrying capacity of the cardiac stroke volume, enabling reduced cardiac output. Unlike in ARDS in which the therapeutic goal is bridge to recovery, the focus for the pre-transplant patient is to maintain viability and functionality and active transplant listing to ensure opportunity to obtain donor organs. Our approach is to optimize patient comfort and the ability to participate in physical therapy. If a patient is adequately supported on ECMO operating within acceptable parameters and oxygen supplementation, we limit volume resuscitation unless indicated for hypotension or evidence of renal insufficiency. Our major effort is to focus care on the patient and not view the ECMO circuit as an entity that requires treatment.
Hypercapnic respiratory failure
Unlike in hypoxic respiratory failure, in which the fraction of venous return entrained in the circuit determines the therapeutic potential of the extracorporeal circuit, ECMO is highly efficient at CO2 removal and is capable of providing full support at significantly lower blood flow rates. Approximately 90% of CO2 is transported in the blood as bicarbonate ion with the remainder transported bound to hemoglobin or as dissolved gas (Figure 4A) (55). The enzyme carbonic anhydrase is present in red blood cells and promotes interconversion between CO2 and bicarbonate (Figure 4B). This enables the extracorporeal circuit to remove physiologically significant amounts at blood flow rates less than 2 L/min by increasing the sweep gas flow rate through the oxygenator. The lower required blood flow rates allow for smaller diameter cannula.

Common management challenges for the pre-lung transplant patient on ECMO
Mobile patients engaged in activity experience sudden swings in intrathoracic pressure and venous return while also shifting cannula location during movement. These factors contribute to variation in flow entrained by the ECMO circuit and dynamically and unpredictably change the provided support. Patients with lung disease also frequently experience coughing, common in CF patients with acute exacerbations and particularly debilitating in patients with IPF, that cause extreme variations in intrathoracic pressure which induce decreases in circuit flow. As venous return decreases, excessive negative pressure in the withdrawal cannula from the pump causes the vena cava to collapse around the cannula and transiently cease flow. The resulting visible recoil in circuit tubing is termed “circuit chugging” and is typically transitory in the awake patient otherwise on stable support.
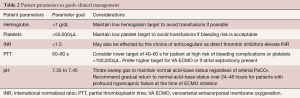
The standard intervention to circuit chugging for patients on ECMO for circulatory support is volume resuscitation with either fluid or blood products. In the case of the awake pre-lung transplant ECMO patient, volume expansion may induce pulmonary edema and worsen gas exchange. Volume management is a particular challenge in this patient population as typical tools used to assess RV preload are difficult to interpret in the setting of VV ECMO. Our preference in management is to be guided by the patient and to tolerate intermittent circuit chugging while working to suppress contributing factors such as coughing. Patients well supported by ECMO and non-invasive supplemental oxygen and who are able to participate in physical therapy with no evidence of end-organ damage, are considered optimized. A recent review detailed clinical aspects of physical therapy in the cardiac ECMO population and details many of the practical considerations relevant to lung transplant patients (56). A summary of treatment parameters for the ECMO-supported patient is shown in Table 2.
Full table
ECMO support for the pre-lung transplant patient with right ventricular failure
Patients with RV dysfunction waiting for lung transplantation present a particularly complex management challenge. Cor pulmonale is a common manifestation of multiple end-stage lung diseases including IPF, COPD, CF and other conditions such as sarcoidosis, in addition to being a defining characteristic of pulmonary arterial hypertension (57). Centers report a variety of extracorporeal strategies for these patients but no optimal approach has yet been identified. Each method presents technical hurdles that require substantial expertise to master and complicate efforts to compare outcomes. Of additional consideration is determining how each approach may affect efforts to determine a patient’s ongoing eligibility for lung transplant in the setting of extracorporeal support.
Veno-arterial ECMO
The percutaneous venoarterial cannulation approach off-loads the RV by shunting blood from the pulmonary to the systemic circulation. To enable ambulation, some centers have employed an “upper-body” approach in which the return cannula is connected to the axillary artery by a graft (58). This approach provides clinician-titrated off-loading of the RV and may be adjusted based on measurable parameters such as systemic hemodynamics and echocardiography assessment of septal positioning. Limitations of this approach include risk of thromboembolism, disruption of systemic ventriculo-vascular coupling and loss of the ability to assess LV function pre-transplant. This method also requires higher flows in the setting of hypoxic respiratory failure, with uncertain effects on mixing in the aorta and uncertain distribution of oxygenated blood in the systemic circulation.
Veno-venous ECMO cannulation with atrial septostomy
This form of cannulation provides a staged approach to intervention and is the performed via percutaneous interventions (59). A double lumen bicaval cannula is placed to provide gas exchange support. For some patients with underlying RV failure, providing oxygenated blood to the pulmonary circulation reduces pulmonary arterial pressures and alleviates underlying RV dysfunction. For patients with progressive parenchymal loss and ongoing RV failure, atrial septostomy provides a means to offload the RV in the setting of elevated right atrial pressure. Coupled with VV ECMO, the septostomy allows the shunting of oxygenated blood across the atrial septum to the circulation while maintaining intact systemic ventriculo-vascular coupling. Safe placement of the septostomy requires simultaneous visualization in two planes using fluoroscopy and transesophageal echocardiography (TEE) for guidance. TEE also enables measurement of the transseptal flow to gauge how large to make the septostomy to ensure adequate flow and sufficient off-loading of the RV.
Right ventricular bypass ECMO
For patients with profound RV failure and hypoxic respiratory failure, an alternative cannulation strategy is to surgically place a withdrawal cannula in the right atrium and to then bypass the RV by placing a return cannula in the pulmonary artery, or to bypass the RV and lungs by placing the return cannula in the pulmonary vein or left atrium. This approach allows for higher flow support due to the large cannula size and central positioning while also allowing for ongoing evaluation of the left ventricle. The limitation of this method is the requirement for thoracic surgery, either via sternotomy or antero-lateral thoracotomy.
Conclusions
ECMO is a powerful extracorporeal support modality capable of providing support for respiratory and circulatory failure, depending on the cannulation strategy. In the setting of rapidly increased adoption, ECMO has become an important means of successfully bridging patients with end-stage lung disease to lung transplantation. Continued improvements in existing technology and new innovations in extracorporeal support will provide clinicians with expanded options to improve pre-transplant care.
Acknowledgments
Funding: SP Keller receives funding from the National Institutes of Health (NIH) 5K08HL143342-02.
Footnote
Conflicts of Interest: SP Keller serves on the Critical Care Advisory Board of Abiomed, Inc., the Scientific Advisory Board of Paragonix Technologies, and consults for X-COR Therapuetics, Inc. He is also a co-founder and serves on the Board of Directors of X-COR Therapeutics, Inc.
References
- Abrams D, Combes A, Brodie D. Extracorporeal membrane oxygenation in cardiopulmonary disease in adults. J Am Coll Cardiol 2014;63:2769-78. [Crossref] [PubMed]
- MacLaren G, Combes A, Bartlett RH. Contemporary extracorporeal membrane oxygenation for adult respiratory failure: Life support in the new era. Intensive Care Med 2012;38:210-20. [Crossref] [PubMed]
- Skilton M, Krishan A, Patel S, et al. Potentiators (specific therapies for class III and IV mutations) for cystic fibrosis. Cochrane Database Syst Rev 2019;1:CD009841. [PubMed]
- Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 2014;370:2071-82. [Crossref] [PubMed]
- Egan TM, Edwards LB. Effect of the lung allocation score on lung transplantation in the United States. J Heart Lung Transplant 2016;35:433-9. [Crossref] [PubMed]
- Valapour M, Skeans MA, Smith JM, et al. OPTN/SRTR 2015 Annual Data Report: Lung. Am J Transplant 2017;17 Suppl 1:357-424. [Crossref] [PubMed]
- Russo MJ, Davies RR, Hong KN, et al. Who is the high-risk recipient? Predicting mortality after lung transplantation using pretransplant risk factors. J Thorac Cardiovasc Surg 2009;138:1234-1238.e1. [Crossref] [PubMed]
- Smits JM, Mertens BJ, Van Houwelingen HC, et al. Predictors of lung transplant survival in eurotransplant. Am J Transplant 2003;3:1400-6. [Crossref] [PubMed]
- Olsson KM, Simon A, Strueber M, et al. Extracorporeal membrane oxygenation in nonintubated patients as bridge to lung transplantation. Am J Transplant 2010;10:2173-8. [Crossref] [PubMed]
- Garcia JP, Kon ZN, Evans C, et al. Ambulatory veno-venous extracorporeal membrane oxygenation: innovation and pitfalls. J Thorac Cardiovasc Surg 2011;142:755-61. [Crossref] [PubMed]
- Fuehner T, Kuehn C, Hadem J, et al. Extracorporeal membrane oxygenation in awake patients as bridge to lung transplantation. Am J Respir Crit Care Med 2012;185:763-8. [Crossref] [PubMed]
- Biscotti M, Gannon WD, Agerstrand C, et al. Awake Extracorporeal Membrane Oxygenation as Bridge to Lung Transplantation: A 9-Year Experience. Ann Thorac Surg 2017;104:412-9. [Crossref] [PubMed]
- Hayanga JW, Lira A, Aboagye JK, et al. Extracorporeal membrane oxygenation as a bridge to lung transplantation: what lessons might we learn from volume and expertise? Interact Cardiovasc Thorac Surg 2016;22:406-10. [Crossref] [PubMed]
- Bartlett RH. Historical Perspectives: Extracorporeal Membrane Oxygenation (ECMO). Neoreviews 2005;6:e251-4. [Crossref]
- Hill JD, O'Brien TG, Murray JJ, et al. Prolonged extracorporeal oxygenation for acute post-traumatic respiratory failure (shock-lung syndrome). Use of the Bramson membrane lung. N Engl J Med 1972;286:629-34. [Crossref] [PubMed]
- Zapol WM, Snider MT, Hill JD, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure: A randomized prospective study. JAMA 1979;242:2193-6. [Crossref] [PubMed]
- Gattinoni L, Kolobow T, Tomlinson T, et al. Low-frequency positive pressure ventilation with extracorporeal carbon dioxide removal (LFPPV-ECCO2R): an experimental study. Anesth Analg 1978;57:470-7. [Crossref] [PubMed]
- Bartlett RH, Roloff DW, Custer JR, et al. Extracorporeal life support: the University of Michigan experience. JAMA 2000;283:904-8. [Crossref] [PubMed]
- Paden ML, Conrad SA, Rycus PT, et al. Extracorporeal Life Support Organization Registry Report 2012. ASAIO J 2013;59:202-10. [Crossref] [PubMed]
- Fineberg HV. Pandemic preparedness and response--lessons from the H1N1 influenza of 2009. N Engl J Med 2014;370:1335-42. [Crossref] [PubMed]
- Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009;374:1351-63. Erratum in: Lancet 2009;374:1330. [Crossref] [PubMed]
- Lorusso R, Alexander P, Rycus P, et al. The Extracorporeal Life Support Organization Registry: update and perspectives. Ann Cardiothorac Surg 2019;8:93-8. [Crossref] [PubMed]
- Palanzo D, Qiu F, Baer L, et al. Evolution of the ECLS circuitry. Artif Organs 2010;34:869-73. [Crossref] [PubMed]
- Lequier L, Horton SB, McMullan DM, et al. Extracorporeal membrane oxygenation circuitry. Pediatr Crit Care Med 2013;14:S7-12. [Crossref] [PubMed]
- Millar JE, Fanning JP, McDonald CI, et al. The inflammatory response to extracorporeal membrane oxygenation (ECMO): a review of the pathophysiology. Crit Care 2016;20:387. [Crossref] [PubMed]
- Maul TM, Massicotte MP, Wearden PD. ECMO Biocompatibility: Surface Coatings, Anticoagulation, and Coagulation Monitoring. In: Extracorporeal Membrane Oxygenation: Advances in Therapy. InTech, 2016. doi: [Crossref]
- Bembea MM, Annich G, Rycus P, et al. Variability in anticoagulation management of patients on extracorporeal membrane oxygenation: an international survey. Pediatr Crit Care Med 2013;14:e77-84. [Crossref] [PubMed]
- Dufour N, Radjou A, Thuong M. Hemolysis and Plasma Free Hemoglobin During Extracorporeal Membrane Oxygenation Support: From Clinical Implications to Laboratory Details. A Review. ASAIO J 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Biffi S, Di Bella S, Scaravilli V, et al. Infections during extracorporeal membrane oxygenation: epidemiology, risk factors, pathogenesis and prevention. Int J Antimicrob Agents 2017;50:9-16. [Crossref] [PubMed]
- Weill D, Benden C, Corris PA, et al. A consensus document for the selection of lung transplant candidates: 2014--an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2015;34:1-15. [Crossref] [PubMed]
- Yusen RD, Edwards LB, Dipchand AI, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-third Adult Lung and Heart-Lung Transplant Report-2016; Focus Theme: Primary Diagnostic Indications for Transplant. J Heart Lung Transplant 2016;35:1170-84. [Crossref] [PubMed]
- Yusen RD, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-first adult lung and heart-lung transplant report--2014; focus theme: retransplantation. J Heart Lung Transplant 2014;33:1009-24. [Crossref] [PubMed]
- Lane CR, Tonelli AR. Lung transplantation in chronic obstructive pulmonary disease: patient selection and special considerations. Int J Chron Obstruct Pulmon Dis. 2015;10:2137-46. [PubMed]
- Ahmadi Z, Bornefalk-Hermansson A, Franklin KA, et al. Hypo- and hypercapnia predict mortality in oxygen-dependent chronic obstructive pulmonary disease: a population-based prospective study. Respir Res 2014;15:30. [Crossref] [PubMed]
- Abdulai RM, Jensen TJ, Patel NR, et al. Deterioration of Limb Muscle Function during Acute Exacerbation of Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med 2018;197:433-49. [Crossref] [PubMed]
- Cuttica MJ, Kalhan R, Shlobin OA, et al. Categorization and impact of pulmonary hypertension in patients with advanced COPD. Respir Med 2010;104:1877-82. [Crossref] [PubMed]
- Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J 2005;26:948-68. [Crossref] [PubMed]
- Kumar A, Kapnadak SG, Girgis RE, et al. Lung transplantation in idiopathic pulmonary fibrosis. Expert Rev Respir Med 2018;12:375-85. [Crossref] [PubMed]
- King TE, Tooze JA, Schwarz MI, et al. Predicting survival in idiopathic pulmonary fibrosis: scoring system and survival model. Am J Respir Crit Care Med 2001;164:1171-81. [Crossref] [PubMed]
- Vianello A, Arcaro G, Molena B, et al. High-flow nasal cannula oxygen therapy to treat acute respiratory failure in patients with acute exacerbation of idiopathic pulmonary fibrosis. Ther Adv Respir Dis 2019;13:1753466619847130. [Crossref] [PubMed]
- Raghu G, Amatto VC, Behr J, et al. Comorbidities in idiopathic pulmonary fibrosis patients: a systematic literature review. Eur Respir J 2015;46:1113-30. [Crossref] [PubMed]
- Elborn JS. Cystic fibrosis. Lancet 2016;388:2519-31. [Crossref] [PubMed]
- Flume PA, Yankaskas JR, Ebeling M, et al. Massive hemoptysis in cystic fibrosis. Chest 2005;128:729-38. [Crossref] [PubMed]
- Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019. [Crossref] [PubMed]
- Curley GF, Laffey JG, Zhang H, et al. Biotrauma and Ventilator-Induced Lung Injury: Clinical Implications. Chest 2016;150:1109-17. [Crossref] [PubMed]
- Suzuki A, Taniguchi H, Ando M, et al. Prognostic evaluation by oxygenation with positive end-expiratory pressure in acute exacerbation of idiopathic pulmonary fibrosis: A retrospective cohort study. Clin Respir J 2018;12:895-903. [Crossref] [PubMed]
- Mollica C, Paone G, Conti V, et al. Mechanical ventilation in patients with end-stage idiopathic pulmonary fibrosis. Respiration 2010;79:209-15. [Crossref] [PubMed]
- Fernández-Pérez ER, Yilmaz M, Jenad H, et al. Ventilator settings and outcome of respiratory failure in chronic interstitial lung disease. Chest 2008;133:1113-9. [Crossref] [PubMed]
- Siuba M, Attaway A, Zein J, et al. Mortality in Adults with Cystic Fibrosis Requiring Mechanical Ventilation. Cross-Sectional Analysis of Nationwide Events. Ann Am Thorac Soc 2019;16:1017-23. [Crossref] [PubMed]
- Tulman DB, Stawicki SP, Whitson BA, et al. Veno-venous ECMO: a synopsis of nine key potential challenges, considerations, and controversies. BMC Anesthesiol 2014;14:65. [Crossref] [PubMed]
- Napp LC, Kühn C, Hoeper MM, et al. Cannulation strategies for percutaneous extracorporeal membrane oxygenation in adults. Clin Res Cardiol 2016;105:283-96. [Crossref] [PubMed]
- Jayaraman AL, Cormican D, Shah P, et al. Cannulation strategies in adult veno-arterial and veno-venous extracorporeal membrane oxygenation: Techniques, limitations, and special considerations. Ann Card Anaesth 2017;20:S11-8. [Crossref] [PubMed]
- Garcia JP, Iacono A, Kon ZN, et al. Ambulatory extracorporeal membrane oxygenation: a new approach for bridge-to-lung transplantation. J Thorac Cardiovasc Surg 2010;139:e137-9. [Crossref] [PubMed]
- Lehr CJ, Zaas DW, Cheifetz IM, et al. Ambulatory extracorporeal membrane oxygenation as a bridge to lung transplantation: walking while waiting. Chest 2015;147:1213-8. [Crossref] [PubMed]
- Arthurs GJ, Sudhakar M. Carbon dioxide transport. Contin Educ Anaesth Crit Care Pain 2005;5:207-10. [Crossref]
- Abrams D, Garan AR, Brodie D. Awake and fully mobile patients on cardiac extracorporeal life support. Ann Cardiothorac Surg 2019;8:44-53. [Crossref] [PubMed]
- Weitzenblum E, Chaouat A. Cor pulmonale. Chron Respir Dis 2009;6:177-85. [Crossref] [PubMed]
- Biscotti M, Vail E, Cook KE, et al. Extracorporeal membrane oxygenation with subclavian artery cannulation in awake patients with pulmonary hypertension. ASAIO J 2014;60:748-50. [Crossref] [PubMed]
- Camboni D, Akay B, Sassalos P, et al. Use of venovenous extracorporeal membrane oxygenation and an atrial septostomy for pulmonary and right ventricular failure. Ann Thorac Surg 2011;91:144-9. [Crossref] [PubMed]






