The impact of hepatitis C viremic donor lung allograft characteristics on post-transplantation outcomes
Introduction
The number of patients with end-stage lung disease who are on the lung transplant waitlist continues to outpace the number of lung transplants performed annually. This imbalance leads to a high mortality on the waitlist. In the United States in 2017, 326 patients with end-stage lung disease died while on the waitlist or were removed from the waitlist due to becoming too sick to undergo transplantation (1). Fortunately lung transplant rates in the US have increased annually from 106.6 per 100 waitlist-years in 2012 to 173.2 per 100 waitlist-years in 2017 (1). This is largely due to having more deceased donors, but also reflects an increase in the number of donor organs used that are recovered. However, lung donor utilization rates remain the lowest of all donor solid organs (2). Strategies are being developed to enable the successful transplantation of deceased-donor organs that historically were discarded due to donor risk factors such as older age, cardiac death, known infections, or behaviors that increase the risk of transmission of infections such as hepatitis C (HCV), hepatitis B, or human immunodeficiency virus (3).
Thoracic organ donors with active HCV were previously declined due to the high risk of HCV transmission and the development of HCV-related complications in the recipient, as well as concerns of poorer post-transplantation outcomes with lower patient and graft survival (4-7). These concerns are being re-evaluated in the era of direct acting antivirals (DAA). DAA have HCV cure rates greater than 97%, are well tolerated, and have limited drug interactions. In the past few years, there are emerging data from single center studies showing the safety and efficacy of transplanting lungs from HCV-infected donors into HCV-uninfected recipients (8). The DONATE HCV Trial reported 44 patients who underwent thoracic organ transplantation at our transplant center (36 lungs and 8 hearts) from HCV viremic donors and were treated with a shortened, four-week course of a pangenotypic DAA, sofosbuvir/velpatasvir, that was initiated a few hours after transplant (9). Of the 35 patients with six months of follow-up at the time of publication, all participants achieved the primary endpoint of sustained virologic response 12 weeks after treatment completion (SVR12) and 6-month graft survival. The recipient outcomes were similar between those who had received organs from HCV-infected donors and HCV-uninfected donors. However, the odds of having acute cellular rejection requiring treatment in the HCV-infected lung cohort were larger than that of the HCV-negative lung cohort.
Mehra and colleagues showed that lung allograft survival does not differ significantly between donors who died from drug intoxication and those who died from other causes (10). Two single center studies have shown that short-term lung allograft outcomes are similar between HCV-infected donors and HCV-uninfected donors (9,11). However, these studies have not assessed whether characteristics of the donor lung allograft at the time of organ offer, including specific patient and organ-related risk factors, differ between HCV-infected and HCV-uninfected donors and impact post-transplantation outcomes.
The objectives of this retrospective study were to: (I) evaluate the baseline donor demographics, clinical risk factors, and allograft-related clinical features including pulmonary function, infection, and chest imaging and bronchoscopy findings between the HCV-viremic donors and HCV-uninfected donors; (II) assess the recipient baseline demographics and clinical risk factors; and (III) analyze the potential impact of these patient specific and organ specific characteristics on post-transplantation clinical outcomes between the two cohorts.
Methods
Patient population and study design
This is a retrospective cohort study that includes adult patients with end-stage lung disease who underwent a single or bilateral lung transplant at Brigham and Women’s Hospital between March 1, 2017 and October 31, 2018 and their corresponding organ donors. Patients were stratified based on their donor HCV status at the time of organ procurement: active HCV infection [HCV nucleic acid amplification test (NAT) positive] or HCV negative [HCV NAT and antibody (Ab) negative]. Recipients of donor lungs who had evidence of prior, resolved HCV infection (HCV Ab positive but NAT negative) were excluded from this analysis.
The transplant recipients of HCV-viremic donor lungs had been enrolled in the DONATE HCV Trial (NCT03086044). Donor acceptance criteria for quality and procurement protocols were the same for HCV NAT positive donors as for HCV-negative donors. HCV-viremic donors were accepted before knowing the HCV genotype and quantitative HCV viral load. Recipients of lungs from HCV-viremic donors received a shortened, pre-emptive four-week regimen of DAA treatment with sofosbuvir (400 mg)/velpatasvir (100 mg), once daily, beginning a few hours post-transplantation. All recipients had an undetectable HCV viral load prior to two weeks and achieved SVR12. Additional details of the DONATE HCV Trial, including the trial protocol, inclusion and exclusion criteria, trial oversight, and initial findings can be found in the 2019 manuscript by Woolley et al. and in the accompanying online Supplementary Appendix (9). This study was approved by our center’s Institutional Review Board and was conducted in collaboration with New England Donor Services.
Data collection
Characteristics of the donor lung allograft at the time of organ offer, including specific patient and organ-related risk factors, were obtained from the United Network for Organ Sharing (UNOS) Standard Transplant Analysis and Research (STAR) database and UNOS DonorNet. The donor arterial partial pressure of oxygen (PaO2) on 100% fraction of inspired oxygen (FiO2) and positive end expiratory pressure (PEEP) 5 cm of water is considered a critical measure of post-transplant allograft function and was assessed for each cohort. Additional allograft clinical features including chest imaging and bronchoscopy findings as well as respiratory cultures were collected. The report of the last chest radiograph and chest computed tomography (CT) study that was available in the DonorNet chart was reviewed for each donor. Findings of significant atelectasis, infiltrates, consolidations, effusions greater than “small” or “trace”, and pneumothoraces were deemed abnormal. CT scans with findings limited to bibasilar atelectasis were considered normal. The donor bronchoscopy reports were reviewed and evidence of purulent or bloody secretions, signs of aspiration or foreign body, and aberrant anatomy were deemed abnormal. Respiratory cultures from the donor hospitals were reviewed and considered positive if pathogens that are not considered normal oral flora grew. Whether the donor was considered to have had a pulmonary infection was a designation made by the donor hospital and was obtained from the UNOS STAR file.
We assessed the medical records of the transplant recipients who underwent a lung transplant during this 20-month period and obtained baseline demographics, clinical data, and transplant outcomes of these patients from our prospectively-maintained DONATE HCV database including length of index hospitalization, readmissions, episodes of acute cellular rejection requiring treatment, graft survival, and patient survival. The lung allocation score is a numerical value from 0 to 100 utilized by UNOS to prioritize lung transplants in the United States based upon highest value. The lung transplant recipients included in this analysis had at least six months of follow-up data.
Statistical analysis
Basic clinical and sociodemographic characteristics were captured descriptively. Continuous variables were summarized with means and standard deviations or medians and ranges or interquartile ranges (IQR). Categorical data were summarized with counts and percentages. We compared sociodemographic and clinical characteristics between the HCV-viremic and HCV-negative cohorts using the Wilcoxon rank-sum test, Student’s t-test, and Fisher exact tests. Statistical analyses were performed using Stata SE 15 version 15.1 (StataCorp LLC, College Station, Texas, USA).
Results
Study cohort
Between March 1, 2017 and October 31, 2018, there were 99 lung transplants performed from either a HCV-viremic donor (n=42) or from a HCV-negative donor (n=57). All 99 patients included in this study had at least 6 months of follow-up and 28 out of 42 (67%) recipients in the HCV-viremic cohort and 49 out of 57 (86%) recipients in HCV-negative cohort had 12-months or more of follow-up time. Baseline donor and recipient sociodemographic and clinical data for the two cohorts are presented in Table 1. Donor age was similar in the two cohorts. The donor cause of death was more often due to anoxia in the HCV-viremic cohort (79% versus 42%, P=0.0001), while more HCV-negative donors died from head trauma (39% versus 14%, P=0.012). The mechanism of the donor’s death, a separate UNOS classification, was more often drug intoxication in the HCV-viremic cohort (71% versus 19%, P=0.0001). Additionally, more HCV-viremic donors had a prior history of cigarette use (83% versus 5%, P=0.0001), illicit drug use within 6 months of death (76% versus 49%, P=0.007), and were considered at increased risk for transmission of human immunodeficiency virus, hepatitis B virus, or HCV as defined by the U.S. Public Health Service (100% versus 21%, P=0.0001) as compared to the HCV-negative cohort.
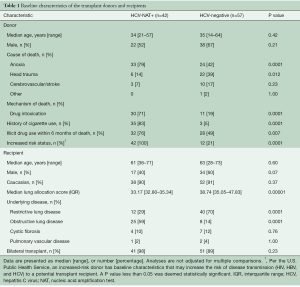
Full table
The age of the recipients was similar in the two cohorts and the majority of recipients in both cohorts underwent a bilateral lung transplant. The median lung allocation score was lower in the HCV-viremic cohort (33.17 versus 38.74, P=0.00001) and a greater proportion of recipients in the HCV-viremic cohort had obstructive lung disease (59% versus 14%, P=0.0001). More recipients in the HCV-negative cohort had restrictive lung disease (70% versus 29%, P=0.0001).
Donor lung allograft-specific clinical features
Overall, the organ-specific clinical characteristics and findings were similar between the HCV-viremic and HCV-negative donors (Table 2). The donor median terminal PaO2 on 100% FiO2 and PEEP 5 cm of water was greater than 400 mmHg and similar in both cohorts as was the mean number of days the donor had been on the ventilator. Analyses of the chest imaging findings revealed that the majority of chest radiograph and CT studies were abnormal and there were no significant differences between the two cohorts. Moreover, the specific abnormalities appreciated on chest CT were equally distributed between the groups. Having a bilateral consolidation was the most commonly appreciated finding in both cohorts. The percentage of bronchoscopy reports with abnormal findings was also similar between the two cohorts. The majority of donors in both cohorts were reported to have had a pulmonary infection and had positive respiratory cultures that were obtained at the donor hospital, of which more than half were Staphylococcus aureus in the HCV-viremic cohort compared to 31% in the HCV-negative cohort.
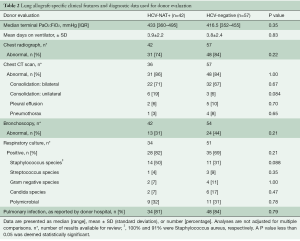
Full table
Transplant recipient outcomes
The recipient outcomes overall were excellent in both cohorts in terms of graft and patient survival at both 6 and 12 months (Table 3). Despite the HCV-viremic cohort having a longer mean donor ischemic time, there were no transplant recipients in the HCV-viremic cohort who had grade 3 pulmonary graft dysfunction at 72 hours. Lung transplant recipients of HCV-negative donors had a significantly longer mean length of hospitalization (12 versus 6 days, P=0.011) as well as a longer mean intensive care unit stay (23 versus 16 days, P=0.019). The odds of having acute cellular rejection requiring treatment were larger in the HCV-viremic cohort at both 6 and 12 months. This finding was not significant and was lower when adjusted for pertinent donor and recipient baseline characteristics in a logistic regression (including the lung allocation score and underlying pulmonary disease of the recipient as well as donor ischemic time and increased risk status of the donor).

Full table
Discussion
The number of lung transplants performed annually is limited by a low utilization rate of donated donor lungs. Efforts to increase acceptance of donated organs include transplanting lungs from HCV-viremic donors into HCV-uninfected recipients. Due to an increasing number of transplants from increased risk donors who have died due to drug intoxication during the past few years, it is critical to understand whether differences in HCV-viremic versus HCV-negative donor and allograft-specific characteristics impact recipient and graft survival.
This study demonstrates that despite a significantly greater proportion of HCV-viremic donors having a history of cigarette use, increased risk status, and dying from anoxia in the setting of drug intoxication, the allograft-specific clinical features as assessed by the proportion of abnormal chest imaging and bronchoscopy findings, positive respiratory cultures, determination of having a pulmonary infection, and the terminal arterial partial pressure of oxygen did not differ between HCV-viremic donors and HCV-negative donors. Despite having differences in baseline demographic and clinical characteristics, the lung transplant recipients in both cohorts had excellent graft and patient survival. Differences in the sociodemographic characteristics and clinical risk factors of the donor did not impact the quality of the allograft at the time of organ procurement or short-term post-transplantation graft and patient survival. However, though it was not statistically significant, there was an increased trend in higher rates of acute cellular rejection requiring treatment in the HCV-viremic cohort.
The evaluation of organ donors is best conceptualized as weighing a constellation of patient-specific and allograft-specific characteristics and their impact on post-transplantation allograft function and overall recipient outcomes. The findings from this study demonstrate that receiving a lung transplant from a donor who died from drug intoxication does not negatively impact graft and recipient survival and further substantiates the results from prior registry studies (10,12,13). The expanding literature supporting the utilization of these increased risk organs, however, often lacks the associated allograft-specific clinical features that are used to determine the quality of the lung itself at the time of organ procurement. The fact that the allograft-specific characteristics did not significantly differ in our study between the HCV-viremic and HCV-negative cohorts illustrates the importance of careful assessment of the quality of the donor organ.
The increased signal of acute cellular rejection in the HCV-viremic lung transplant recipients highlights the need for longer-term follow-up to assess the potential effect of this on the incidence of chronic lung allograft dysfunction. Rejection rates were attenuated in this larger cohort compared to the findings in the initial 2019 DONATE HCV manuscript (9). Further research on the use of HCV-viremic pulmonary allografts in the era of direct-acting antiviral therapy and longer-term outcomes is important as we further expand this donor pool.
Our study is somewhat limited by its small, retrospective nature, hence, conclusions based on these data should be made with caution. Other limitations of this work include its single-center nature, the need to rely on the reports of the bronchoscopy and chest imaging findings rather than being able to review the primary data ourselves, and limited follow-up time which does not allow for the longer-term assessment of chronic lung allograft dysfunction.
These data establish that thoracic organs from HCV-viremic donors have similar organ quality and function at the time of procurement and can be transplanted safely into HCV-negative recipients, with excellent graft and patient survival at 6 and 12 months. Decisions whether to accept thoracic organs from HCV-viremic donors, increased risk donors who have died from drug intoxication, and donors with history of cigarette and drug use are complex and need to be considered in relation to the increased morbidity and mortality associated with longer waiting times on the lung transplant waitlist for alternative donor organs.
Acknowledgments
We thank the patients, organ donors, and their families; the United Network for Organ Sharing and New England Donor Services; and all the members of our multidisciplinary medical and surgical transplant staff at Brigham and Women’s Hospital. Supported in part by the Mendez National Institute of Transplantation Foundation.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Valapour M, Lehr CJ, Skeans MA, et al. OPTN/SRTR 2017 Annual Data Report: Lung. Am J Transplant 2019;19 Suppl 2:404-84. [Crossref] [PubMed]
- Singh E, Schecter M, Towe C, et al. Sequence of refusals for donor quality, organ utilization, and survival after lung transplantation. J Heart Lung Transplant 2019;38:35-42. [Crossref] [PubMed]
- Tullius SG, Rabb H. Improving the Supply and Quality of Deceased-Donor Organs for Transplantation. N Engl J Med 2018;378:1920-9. [Crossref] [PubMed]
- Pereira BJG, Milford EL, Kirkman RL, Levey AS. Transmission of Hepatitis C Virus by Organ Transplantation. N Engl J Med 1991;325:454-60. [Crossref] [PubMed]
- Haji SA, Starling RC, Avery RK, et al. Donor hepatitis-C seropositivity is an independent risk factor for the development of accelerated coronary vasculopathy and predicts outcome after cardiac transplantation. J Heart Lung Transplant 2004;23:277-83. [Crossref] [PubMed]
- Carreno MC, Piedad UG, Maite L, et al. Hepatitis C virus infection after lung transplantation: dim prognosis. J Heart Lung Transplant 2001;20:224. [Crossref] [PubMed]
- Englum BR, Ganapathi AM, Speicher PJ, et al. Impact of donor and recipient hepatitis C status in lung transplantation. J Heart Lung Transplant 2016;35:228-35. [Crossref] [PubMed]
- Khan B, Singer LG, Lilly LB, et al. Successful Lung Transplantation From Hepatitis C Positive Donor to Seronegative Recipient. Am J Transplant 2017;17:1129-31. [Crossref] [PubMed]
- Woolley AE, Singh SK, Goldberg HJ, et al. Heart and Lung Transplants from HCV-Infected Donors to Uninfected Recipients. N Engl J Med 2019;380:1606-17. [Crossref] [PubMed]
- Mehra MR, Jarcho JA, Cherikh W, et al. The Drug-Intoxication Epidemic and Solid-Organ Transplantation. N Engl J Med 2018;378:1943. [Crossref] [PubMed]
- Cypel M, Feld JJ, Galasso M, et al. Prevention of viral transmission during lung transplantation with hepatitis C-viraemic donors: an open-label, single-centre, pilot trial. Lancet Respir Med 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Whited WM, Ising MS, Trivedi JR, et al. Use of drug intoxicated donors for lung transplant: Impact on survival outcomes. Clin Transplant 2018;32:e13252. [Crossref] [PubMed]
- Durand CM, Bowring MG, Thomas AG, et al. The drug overdose epidemic and deceased-Donor transplantation in the United States a national registry study. Ann Intern Med 2018;168:702. [Crossref] [PubMed]






