Outcome of rapid deployment aortic valves: long-term experience after 700 implants
Introduction
Surgical aortic valve replacement (SAVR) with a rapid-deployment (RD) bioprosthesis was a major step forward in the surgical armamentarium in terms of facilitating a minimally invasive approach and ensuring superior hemodynamic performance in comparison with other surgical sutured valves (1,2). As minimally invasive AVR is mostly associated with longer cross-clamp times due to more difficult valve exposure, RD valves may overcome the prolonged times necessary for suture placement (3). In the era of transcatheter technologies, when the indication for transcatheter aortic valve replacement (TAVR) has been gradually extended to low-risk populations (4), RD systems might be a superior alternative in this patient population in terms of hemodynamics, but also in complex combined procedures (5,6). Our institution participated in the market-release trial of the Edwards Intuity Valve System, which was standardized at our department. After our initial report of 500 Intuity implantations at our center, we now report the updated 9-year single-center experience with the Edwards Intuity Valve regarding mortality, re-intervention or re-operation with valve explantation rates, valve-related adverse events and hemodynamic performance (7).
Methods
Study population
We previously published the study population described herein in detail (7). For this updated analysis, 700 patients included from 2010 to May 2019 were assessed out of the Vienna Intuity Comprehensive Evaluation (VICE) registry. The registry was approved by the Institutional Review Board (1861/2016) and patients signed the informed consent either pre- or post-operatively. The safety and effectiveness of this bioprosthesis was assessed. Moreover, all failed implantations were analyzed, but the patients were not included in further analysis if another prosthesis was implanted.
All patients were included in this analysis and followed-up according to our institutional protocol. This consisted of a clinical follow-up at discharge, 3 months, 1, 3, 5 and 10 years, which evaluates the clinical status and occurrence of any adverse events, as well as hemodynamic performance by transthoracic echocardiography (TTE) and electrocardiogram. At 2, 4 and 7 years after the index procedure, a telephone follow-up is performed. The follow-up time was up to 9 years, with a mean of 28±23 months and a median of 19 [11–41] months.
Study endpoints
We primarily assessed short and long-term survival as well as structural/non-structural valve dysfunction and hemodynamic valve performance by TTE (8,9). Furthermore, all recommended clinical outcomes for reporting results after surgical valve replacement were assessed as secondary endpoints (8).
All deaths after valve implantation were assessed for the calculation of overall mortality. Early mortality (death occuring during the first 30 postoperative days) and in-hospital mortality (any event occurring between surgery and first discharge) was reported. We applied the EuroScore II (European system for cardiac operative risk evaluation) and STS score (Society of Thoracic Surgeons Score) for risk assessment.
Valve-related adverse events (e.g., structural valve degeneration, non-structural valve deterioration, endocarditis, valve thrombosis, major bleeding events, thromboembolic events, myocardial infarction and pacemaker implantation) were assessed according to current guidelines (8). Further events were categorized and analysed as previously described (7).
Statistical analysis
Continuous variables are shown as mean and standard deviations or median (25th–75th interval). Total numbers and proportions are reported for categorical outcomes. The Kaplan-Meier and lifetable methods are performed to assess survival and valve related events. The safety endpoints are reported as early (≤30 postoperative days) or late (>30 postoperative days) events. Early events are reported as numbers and percentages and late events as linearized event rates per patient year (%ppy) of follow-up and calculated as cumulative number of late events divided by the total patient-years. Statistical calculations comparing continuous variables are made using a Wilcoxon-Mann-Whitney test; comparisons of categorical variables are made using Pearson’s chi-square test or ANOVA when more than two groups are compared. IBM SPSS Statistics 25 (IBM Corp. Released 2016. IBM SPSS Statistics for Mac, Version 25.0., IBM Corp., Armonk, NY, USA) was used for statistical analysis. A P value of less than 0.05 is considered as significant.
Results
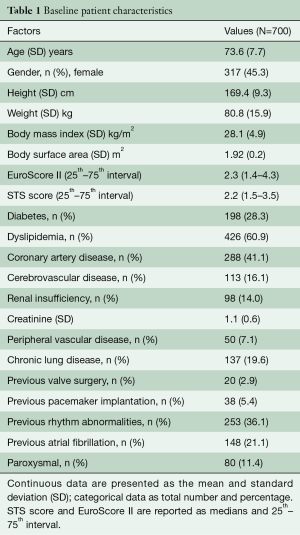
We report the results of 700 patients who underwent surgical aortic valve replacement with the Intuity valve system from the first implant in 2010 until May 2019 at our department. Patients had a mean age of 73.6 [standard deviation (SD): 7.7] years, and 317 (45.3%) female patient were included. Sixty-seven percent were in NYHA functional class III or IV. EuroScore II and STS score were 2.3 (1.4–4.3) and 2.2 (1.5–3.5), respectively (Table 1).
Full table
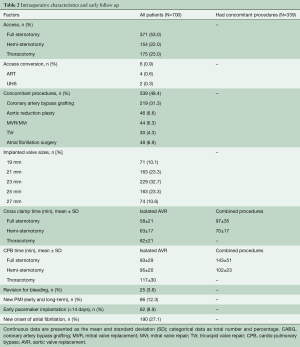
All fifteen adult cardiac staff surgeons performed the implants; ten surgeons (67%) performed a minimum of ten implantations. Concomitant procedures were carried out in 339 (48.4%) patients. Coronary artery bypass grafting (CABG) was performed in 219 (31.3%) patients; other procedures are detailed in Table 2. A median sternotomy was used in 371 (53%) surgeries, including concomitant procedures; the remaining patients were operated through a minimally invasive approach, either through an upper-hemisternotomy in 154 (22%) cases, or anterior right thoracotomy in 175 (25%) patients; six patients required conversion to full sternotomy (FS) (0.9%). When all patients including combined procedures were analyzed, the cardio-pulmonary bypass (CPB) and cross-clamp times were 120±44 and 81±31 minutes (Table 2). We performed central cannulation in most of our minimally invasive procedures (Table 2). The valve deployment was not successful during the initial approach and required a second valve in 15 patients (2.1%).
Full table
Implantation failures
In addition to the 700 successful implantations, another 15 patients were switched to a conventional valve after one or more failed RD-AV implantation attempts. This was attributed to a large RD prosthesis, which could not be placed in the annulus in two of these patients, while ten patients had a pop-out, and one patient was observed to have atrioventricular dehiscence after decalcification of the mitral-aortic continuity at the time of valve positioning. Another patient had severe paravalvular regurgitation (PVR) after clamp removal and in the last case, one nadir stich teared through the annulus and by the second stich, with the valve already positioned in the annulus, one of the prosthesis leaflets had been accidentally injured.
Clinical outcomes
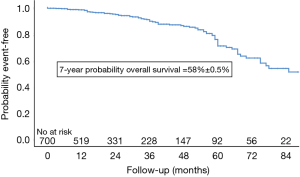
Five patients died during the first 30 days (0.7%). Peri-operative mortality occurred in in 10 (1.4%) patients after AVR with concomitant procedures due to: cardiac arrest due to asystole, refractory to re-animation on 6th postoperative day (n=1), cardiogenic shock with cardiac arrest (n=1) on day 53, multi-organ failure (n=3) on day 4, 92 and 111 post-operatively, ARDS (n=1, 0.2%) on day 34, acute kidney failure (n=1, 0.2%) on day 149, gastro-intestinal bleeding with cardiogenic shock on day 19, sepsis with Candida albicans and respiratory insufficiency by pneumonia on day 31, and lastly, cardiogenic shock with re-operation by severe paravalvular leak with valve explantation and extracorporeal membrane oxygenation (ECMO) as well as diffuse uncontrollable bleeding on day 30. Overall, 83 patients died (12.1%). Long-term survival was 98%, 91%, 76% and 58% at 1, 3, 5 and 7 years after surgery (Figure 1).
Neurologic outcome
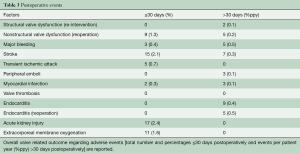
Early stroke occurred in 15 (2.1%) patients; 7 (0.33%ppy) late events were reported (Table 3).
Full table
Early revision and bleeding
There were 3 (0.4%) early bleeding events and 11 (1.6%ppy) late major bleeding events. Another 25 (3.6%) patients required early peri-operative revision for bleeding.
Re-intervention and valve dysfunction
Nine patients (1.3%) were re-operated within 30 days of the index operation and an additional 12 patients (0.56%ppy) required late re-interventions or re-operations with valve explantation (see Table 3 and Figure 2).
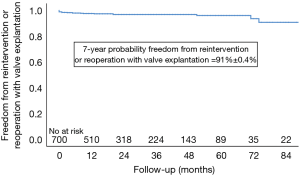
The nine early re-operations took place on the following postoperative days: 1, 5, 7, 8, 9, 15, 26, 29, and were necessary due to severe PVR in eight cases and due to septal rupture and acute bleeding in a patient in whom a myectomy was performed (Table 3); another 5 patients underwent valve explantation for non-structural dysfunction, four patients due to progressive PVR on days 154, 186, 272, 625 and one patient on day 49 with structural damage of the mitral valve apparatus and an intact aortic valve.
Another two patients (0.1%ppy) received a valve-in-valve (ViV) procedure at 52 and 75 months after the SAVR for structural degeneration.
Re-operation with valve explanation occurred, and 5 (0.2%ppy) late cases of endocarditis were reported at 4, 10, 13, 20 and 73 months after the index procedure.
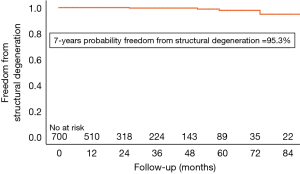
Structural valve degeneration occurred in 4 (0.2%ppy) patients, 2 (0.1%ppy) underwent a ViV procedure and another one died after cardiac decompensation and cardiogenic shock (Figure 3). Mild PVR was observed in 47 (6.7%) of patients, moderate PVR was observed in 15 (2.1%) patients, and severe PVR was observed in 8 (1.1%) patients. Patients who developed symptoms related to PVR were operated on as described above.
Endocarditis
Nine patients (0.4%ppy) suffered from postoperative endocarditis, of which 5 (0.2%ppy) underwent re-operation and valve explantation as described above.
Pacemaker implantation
A pacemaker implantation was required in 61 patients (8.9%, and 9.4% if patient with preoperatively implanted pacemakers are excluded) during the first 14 postoperative days.
Hemodynamic performance
The mean gradients at discharge, 1, 3 and 5 years follow-up were 13±5, 11±4, 12±5 and 13±8 mmHg. The effective orifice area (EOA) and indexed EOA at discharge were 1.89 (SD: 0.57) cm2 and 0.99 (SD: 0.28) cm/m2. Severe prosthesis-patient mismatch (PPM) occurred in 24 patients (3.4%).
Discussion
Minimally invasive SAVR with this RD-AV gradually became the treatment of choice for isolated AVR with 78% patients operated through minimal access—either an upper-hemisternotomy or anterior right thoracotomy. As previously reported (7), during our learning curve, we started implanting this valve through a standard full sternotomy and subsequently shifted to minimally invasive approaches. Our preliminary results (2) demonstrated the relatively quick learning curve, which subsequently facilitated the minimally invasive approaches. As previously reported, we are in favour of direct arterial cannulation also in minimally invasive cases to reduce groin complications (10).
The subvalvular stent fixation and the absence of any pledget material might reshape the outflow tract and improve transvalvular hemodynamics, with superior gradients and reduced turbulent flow (11), in comparison to the conventional valve of the same manufacturer (1,12). Fallon and colleagues (13) demonstrated the occurrence of PPM in up to 65% of patients documented in the STS database requiring an isolated AVR, with survival rates being significantly reduced for any degree of PPM (moderate to none: HR, 1.08; 95% CI, 1.05 to 1.12; severe to none: HR, 1.32; 95% CI, 1.25 to 1.39), and 10-year adjusted survival rates of 46%, 43%, and 35% for none, moderate, and severe PPM, respectively (P<0.001). Other studies showed a reduced incidence of PPM after RD-AVR, compared with conventional valves (12-14). Our single-center large experience confirms the low incidence of PPM after RD-AVR, as moderate and severe PPM occurred in 13% and 3% of cases, and was also low in patients with a small aortic root (15).
Residual moderate-severe PVR was observed in 3% of cases. In order to reduce the incidence of PVR, we were cautious and rather reluctant to implant this bioprosthesis in patients with extensive calcifications of the root and rigid sinuses or true bicuspid aortic valves (Sievers type 0). The rate of structural valve degeneration was very low, and RD valves are an ideal target for ViV procedures (16,17).
We report a total rate of 8.9% early new pacemaker implantation, which is higher than in other surgical valves (18). The meta-analysis from the Sutureless and Rapid-Deployment Aortic Valve Replacement International Registry (SURD-IR) (19) also showed a pacemaker rate of 10.4%. We recently published a detailed analysis of postoperative conduction disturbances of this population (20). Our intermediate-long term results did not show any significant influence on overall survival related to pacemaker implantation.
Study limitations
This study combines the results of pre-market clinical trials and a post-market registry. Therefore, patient population was not selected and our results included the learning curve for 15 surgeons.
Conclusions
We report excellent long-term results in this updated single center experience for rapid-deployment aortic valves regarding durability, safety and hemodynamic performance.
Acknowledgments
The patients from multicenter clinical trials funded by Edwards Lifesciences (Irvine, CA, USA) were included in this analysis. Our institution receives financial support from the same company to conduct a long-term follow up after Intuity valve implantation, and to maintain a long-term follow-up registry.
Footnote
Conflicts of Interest: AK: Speakers honoraria from Edwards Lifesciences. MA: Research Grant from Edwards Lifesciences, Proctor (Abbott, Edwards), Advisor (Medtronic). GL: Consulting fees from Edwards Lifesciences. TH: Research Grant Edwards Lifesciences. The other authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Andreas M, Wallner S, Habertheuer A, et al. Conventional versus rapid-deployment aortic valve replacement: a single-centre comparison between the Edwards Magna valve and its rapid-deployment successor. Interact Cardiovasc Thorac Surg 2016;22:799-805. [Crossref] [PubMed]
- Borger MA, Moustafine V, Conradi L, et al. A randomized multicenter trial of minimally invasive rapid deployment versus conventional full sternotomy aortic valve replacement. Ann Thorac Surg 2015;99:17-25. [Crossref] [PubMed]
- Berretta P, Andreas M, Carrel TP, et al. Minimally invasive aortic valve replacement with sutureless and rapid deployment valves: a report from an international registry (Sutureless and Rapid Deployment International Registry)†. Eur J Cardiothorac Surg 2019;56:793-9. [Crossref] [PubMed]
- Mack MJ, Leon MB, Thourani VH, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med 2019;380:1695-705. [Crossref] [PubMed]
- Kocher AA, Laufer G, Haverich A, et al. One-year outcomes of the Surgical Treatment of Aortic Stenosis with a Next Generation Surgical Aortic Valve (TRITON) trial: a prospective multicenter study of rapid-deployment aortic valve replacement with the EDWARDS INTUITY Valve System. J Thorac Cardiovasc Surg 2013;145:110-5; discussion 115-6. [Crossref] [PubMed]
- Shrestha M, Folliguet TA, Pfeiffer S, et al. Aortic valve replacement and concomitant procedures with the Perceval valve: results of European trials. Ann Thorac Surg 2014;98:1294-300. [Crossref] [PubMed]
- Andreas M, Coti I, Rosenhek R, et al. Intermediate-term outcome of 500 consecutive rapid-deployment surgical aortic valve procedures†. Eur J Cardiothorac Surg 2019;55:527-33. [Crossref] [PubMed]
- Akins CW, Miller DC, Turina MI, et al. Guidelines for reporting mortality and morbidity after cardiac valve interventions. Eur J Cardiothorac Surg 2008;33:523-8. [Crossref] [PubMed]
- Zoghbi WA, Chambers JB, Dumesnil JG, et al. Recommendations for evaluation of prosthetic valves with echocardiography and doppler ultrasound: a report From the American Society of Echocardiography's Guidelines and Standards Committee and the Task Force on Prosthetic Valves, developed in conjunction with the American College of Cardiology Cardiovascular Imaging Committee, Cardiac Imaging Committee of the American Heart Association, the European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography and the Canadian Society of Echocardiography, endorsed by the American College of Cardiology Foundation, American Heart Association, European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography, and Canadian Society of Echocardiography. J Am Soc Echocardiogr 2009;22:975-1014. [Crossref] [PubMed]
- Andreas M, Mahr S, Kocher A, et al. Minimalinvasiver Aortenklappenersatz über eine anteriore rechtsseitige Thorakotomie. Zeitschrift für Herz- Thorax-und Gefäßchirurgie 2017;31:241-6. [Crossref]
- Capelli C, Corsini C, Biscarini D, et al. Pledget-armed sutures affect the haemodynamic performance of biologic aortic valve substitutes: a preliminary experimental and computational study. Cardiovasc Eng Technol 2017;8:17-29. [Crossref] [PubMed]
- Wahlers TCW, Andreas M, Rahmanian P, et al. Outcomes of a rapid deployment aortic valve versus its conventional counterpart: a propensity-matched analysis. Innovations (Phila) 2018;13:177-83. [Crossref] [PubMed]
- Fallon JM, DeSimone JP, Brennan JM, et al. The incidence and consequence of prosthesis-patient mismatch after surgical aortic valve replacement. Ann Thorac Surg 2018;106:14-22. [Crossref] [PubMed]
- Laufer G, Haverich A, Andreas M, et al. Long-term outcomes of a rapid deployment aortic valve: data up to 5 years. Eur J Cardiothorac Surg 2017;52:281-7. [Crossref] [PubMed]
- Coti I, Haberl T, Scherzer S, et al. Rapid-Deployment Aortic Valves for Patients With a Small Aortic Root: A Single-Center Experience. Ann Thorac Surg 2020. [Epub ahead of print]. [PubMed]
- Landes U, Dvir D, Schoels W, et al. Transcatheter aortic valve-in-valve implantation in degenerative rapid deployment bioprostheses. EuroIntervention 2019;15:37-43. [Crossref] [PubMed]
- Andreas M, Coti I, Laufer G, et al. Valve-in-valve transcatheter aortic valve implantation into a novel, sutureless bioprosthesis: technical considerations. EuroIntervention 2018;13:1902-3. [Crossref] [PubMed]
- Greason KL, Lahr BD, Stulak JM, et al. Long-term mortality effect of early pacemaker implantation after surgical aortic valve replacement. Ann Thorac Surg 2017;104:1259-64. [Crossref] [PubMed]
- Di Eusanio M, Phan K, Berretta P, et al. Sutureless and Rapid-Deployment Aortic Valve Replacement International Registry (SURD-IR): early results from 3343 patients. Eur J Cardiothorac Surg 2018;54:768-73. [Crossref] [PubMed]
- Coti I, Schukro C, Drevinja F, et al. Conduction disturbances following surgical aortic valve replacement with a rapid-deployment bioprosthesis. J Thorac Cardiovasc Surg 2020. [Epub ahead of print]. [Crossref] [PubMed]