Frozen elephant trunk with straight vascular prosthesis
Introduction
Disease of the aorta has many possible permutations and combinations. As blood courses from the heart to the peripheries, it supplies the vital organs—brain, spinal cord, and viscera. Furthermore, the anatomy of the aorta extends anterior-posterior, cephalo-caudal, right-left makes it a challenging organ to address for aortic surgeons. The frozen elephant trunk (FET) enables surgical access to the ascending aorta, aortic arch, and the descending thoracic aorta via a median sternotomy, facilitating the ability to address a wide range of aortic pathology.
Classic is frozen—the changing paradigm
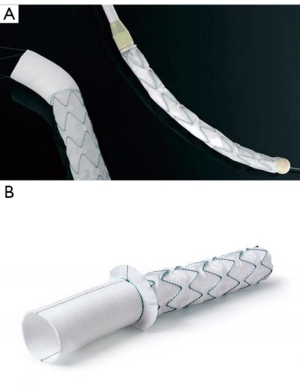
Multi-segmental aortic disease involving the ascending aorta, aortic arch, and the descending aorta has been historically managed in two stages. Several attempts were made to simplify and perform a single-stage procedure. Hans Borst with his colleagues introduced the elephant trunk (cET) nearly four decades ago (1). Modifications were suggested by Crawford and Svensson (2,3), however, it took several years before it became routine clinical application in high-volume centers. The cardinal considerations regarding the cET dwell in the cumulative risk of two major surgical procedures and the inevitable interval mortality. Further literature suggests that nearly a third to half of the patients do not come back for a second surgery—either due to refusing surgery, dead at home, or lost to follow-up (4). Several innovations and attempts were made by different surgeons to prevail over this enigma to find a lasting one-stage repair including distal aortic remodeling and a perfect landing zone for secondary interventions. Following pursuit, endovascular stenting was introduced in combination with classical arch surgery (5,6). Modifying this new operative technique, the Essen team consisting of myself and Drs. Ulf Herold and Konstantinos Tsagakis started using self-expanding aortic stent grafts (Talent®) mounted in reversed fashion. These grafts were introduced into the descending aorta from the opened arch before ascending/arch replacement. This was the purpose of the one-stage aortic repair since 2001 (7,8). Not disheartened by the results, we followed pursuit and developed the ‘Essen I prosthesis’ which consisted of polyester fabric with an extremely flexible z-shaped Nitinol wire skeleton and incorporated a Dacron prosthesis of 7 cm proximally (9) (Figure 1A). In Japan and as of March 2008, 60 patients underwent implantation of the Frozenix J graft which has a unique double-layered oval-shaped nitinol stent that easily conforms to the curvature of the aorta (10). In 2010, implantation of the Vascutek Thoraflex hybrid graft (Vascutek, Scotland) commenced and the results of the first 34 patients were published in 2012 (11). Collectively, this started a new era of the conventional elephant trunk ‘freezing’ to a considerable extent.
“Aorto-isation” of Essen
The advent of FET, saw the gradual reduction of cET in many large volume centers. “Essen I Prosthesis” was further developed into Evita Open and later Evita Open Plus (Jotec®, Hechingen, Germany), becoming the first commercially available FET in 2005 (Figure 1B). This combined the advantage of open surgery couple with endovascular intervention. The collar allowed ease of suturing, and the hemostasis was further augmented by the endovascular stent graft. Our team in Essen continued to develop and modify operative techniques paralleling the advancement in the device technology and into the FET surgical era, hence reducing morbidity and mortality.
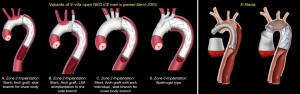
We previously described a modified approach for direct cannulation of the true lumen of the ascending aorta in very acute type A dissection, consisting of primary venous exsanguination and ascending aortic cannulation of the true lumen under direct vision with controlled de-airing (12). Angioscopy (using a flexible video scope) was introduced into FET surgery as an important adjuvant tool for real-time decision-processing as to whether or not the distal aorta warranted further intervention with a hybrid stent-graft (13). Furthermore, the angioscope was an adjunct tool in our armamentarium to evaluate additional re-entry tears, control positioning of the stent-graft, and aid in the decision making on additional stent-graft utilization. Our performance and applicability of the angioscope were further extended to entail aortic valve surgery (14). A major concern of FET was the higher incidence of paraplegia compared to the cET. The incidence of paraplegia had prompted the reduction of the length of the stent-graft to 130 mm (15). Progressively FET surgery attained the proximalization of the distal anastomosis to zone 2 rather than zone 3 (16). This was associated with decreased incidences of cardiac arrest, selective cerebral perfusion, and visceral ischemia. It further helped to reduce the incidence of recurrent nerve palsy, the ease of anastomosis, and control of hemorrhage. This approach mandated the ligation of the left subclavian artery at its origin; reconstruction with an extra-anatomical bypass from the left axillary artery (LAA) using an 8 mm vascular graft (17). In a recent systematic review and meta-analysis of 2,161 patients (1,919 E-vita and 242 Thoraflex) suggested that mortality and morbidity are lower in thoracic aortic aneurysm surgery for the E-vita device than for Thoraflex (18).
Indications of FET
FET is indicated in distal aortic arch aneurysms, aneurysm of the proximal and the descending thoracic aorta, chronic type B aortic dissection, residual dissection after the proximal aortic repair and acute type I aortic dissection. Though the use of FET in acute type I aortic dissection is still a matter of debate, we believe the use of FET is of immense benefit for this population cohort given a concomitant distal arch or proximal descending aortic tear.
Additionally, FET can be used in patients with complicated Type III aortic dissection, including retrograde dissection in zone 2, short landing zone (<2 cm) for TEVAR in zone 2, or diameter of aortic arch >40 mm. FET is also indicated in Type Ia endoleak after TEVAR or in patients with a stent-induced new entry (SINE). In a study from Essen, an angioscope was used in 124 patients to identify the position and morphology of distal re-entry sites in type I/III AD patients. This showed that there was re-entry in 73% of patients 5 cm distally to the origin of the left subclavian artery (LSA) and 31% in the 6–10 cm range (19). The 130 mm Evita stent-graft covers both these re-entry points.
Extracorporeal circuit for FET
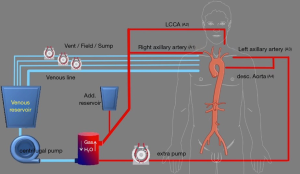
With the advancements in techniques, the extracorporeal circuit was adjusted. The right axillary artery (A1) and a Y-line is added for future left common carotid perfusion (A2) (Figure 2). The second pump is connected to the LAA through an 8 mm tube graft (A3). A Y-line is added to this for perfusion of the downstream aorta at a later stage (A4). The 8 mm graft anastomosed to the LAA is brought into the mediastinum through the left first intercostal space. When the temperature reaches 28 °C, bilateral antegrade cerebral perfusion is established with A1 & A2. The brain perfusate is cooled to 22 °C. The LSA is ligated at the origin and perfusion through A3 is started. The Evita Open Plus is inserted in the descending thoracic aorta and fixed to the distal arch. A balloon-tipped Foley catheter is taken into the Evita graft for lower body perfusion (A4). The supra-aortic arch vessels are reimplanted on to the arch graft and the patient is rewarmed.
Results at Essen
Over 13 years, from February 2005 to October 2018, the Evita Open has been implanted in the 307 patients (20). The mean age was 59±12 years with a male predominance of 69%. Acute aortic dissection (AAD) constitutes 55% of these patients. Additionally, 37% of the patients underwent root replacement, Bentall or David procedure, and 24% required coronary artery bypass grafting.
The 30-day mortality was 12%, the incidence of permanent stroke was 7% and paraplegia was 3%. The 5-year the survival rate for AAD, CAD, and TAA (thoracic aortic aneurysm) were 67%, 74%, 65% respectively. The overall 8-year survival was 60%. The overall freedom for aortic-related death at 10 years was 91%. On comparing Zone 3 with Zone ≤2 implantations of Evita graft, most recent results indicate that the 5-year and 8-year survival significantly favors Zone 2 implantations (75% vs. 60% and 74% vs. 52%, P<0.05). With a near 100% follow-up, comparing FET with proximal repair in acute Type I aortic dissection, freedom from reintervention at 5- and 10-year favors FET significantly (87% vs. 68% and 74% vs. 48%, respectively). This corresponds well with false lumen thrombosis and positive remodeling around the descending stent-graft in AAD of 90% versus 78% in CAD. Further downstream, annual, or biannual follow-up is warranted to identify patients with progressive aortic growth. In a degenerative aneurysm, it could be excluded 100% of the time, when restricted to the distal arch and proximal descending aorta (20).
Future perspectives
E-vita Open NEO (Figure 3): this comes in three variants (I) a stent and an arch graft with a side branch for lower body perfusion; (II) a stent graft and the arch graft having individual branch grafts for selective anastomosis to the supra-aortic arch vessels; (III) Spielvogel type: this has a ‘no-arch-touch’ principle with the suture line in Zone 0. The length of the stent-graft varies from 16–19 cm. There is a trifurcated graft to perfuse the supra-aortic arch vessels adjacent to a separate perfusion port for the lower body. The introducer is shorter with maximum flexibility for ease of surgery. All these measures will help to shorten the ischemia time. CE-mark has been granted in March 2020 and is soon to be available in Europe.
E-Novia (Figure 3): this prototype is to be considered for very acute type I aortic dissection, Penn B, C, BC, and patients with severe concomitant disease. It will have a covered and non-covered stent-graft portion. The covered stent-graft will be in the descending thoracic aorta and non-covered stent-graft in the aortic arch. The anastomosis would be performed in Zone 0, thus reducing ischemic times as well as hypothermic circulatory arrest time dramatically. The first clinical results are being published soon (21).
Conclusions
Over the past two decades, aortic surgery procured an outstanding surgical and clinical applicability thanks to the innovative and technological advancement coupling surgical skills and modification with industry and tailored engineering. Precision in surgical validation and performance with focused and sustainable leadership as produced in Essen has revolutionized our results in thoracic aortic aneurysm surgery albeit in elective and non-elective settings.
The E-Vita Open Plus, E-Novia, and E-Vita Open NEO will represent a family of graft variations, enabling us to deal with all kinds of pathologies of the arch aneurysms and pathology through combined open endovascular approach.
Acknowledgments
None.
Footnote
Conflicts of Interest: Dr. HJ is consultant to CryoLife/JOTEC, receives speaker’s honoraria, and has received royalties until December 2019. The other authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Borst HG, Walterbusch G, Schaps D. Extensive aortic replacement using "elephant trunk" prosthesis. Thorac Cardiovasc Surg 1983;31:37-40. [Crossref] [PubMed]
- Crawford ES, Coselli JS, Svensson LG, et al. Diffuse aneurysmal disease (chronic aortic dissection, Marfan, and mega aorta syndromes) and multiple aneurysm. Treatment by subtotal and total aortic replacement emphasizing the elephant trunk operation. Ann Surg 1990;211:521-37. [Crossref] [PubMed]
- Svensson LG. Rationale and technique for replacement of the ascending aorta, arch, and distal aorta using a modified elephant trunk procedure. J Card Surg 1992;7:301-12. [Crossref] [PubMed]
- Shrestha M, Martens A, Krüger H, et al. Total aortic arch replacement with the elephant trunk technique: single-centre 30-year results. Eur J Cardiothorac Surg 2014;45:289-95; discussion 295-6. [Crossref] [PubMed]
- Kato M, Ohnishi K, Kaneko M, et al. New graft-implanting method for thoracic aortic aneurysm or dissection with a stented graft. Circulation 1996;94 Suppl:II188-93. [PubMed]
- Suto Y, Yasuda K, Shiiya N, et al. Stented elephant trunk procedure for an intensive aneurysm involving distal aortic arch and descending aorta. J Thorac Cardiovasc Surg 1996;112:1389-90. [Crossref] [PubMed]
- Herold U, Piotrowski J, Baumgart D, et al. Endoluminal stent graft repair for acute and chronic type B aortic dissection and atherosclerotic aneurysm of the thoracic aorta: an interdisciplinary task. Eur J Cardiothorac Surg 2002;22:891-7. [Crossref] [PubMed]
- Eggebrecht H, Baumgart D, Schmermund A, et al. Endovascular stent-graft repair for penetrating atherosclerotic ulcer of the descending aorta. Am J Cardiol 2003;91:1150-3. [Crossref] [PubMed]
- Jakob H, Tsagakis K, Leyh R, et al. Development of an integrated stent graft-dacron prosthesis for intended one-stage repair in complex thoracic aortic disease. Herz 2005;30:766-8. [Crossref] [PubMed]
- Uchida N, Katayama A, Higashiue S, et al. A new device as an open stent graft for extended aortic repair: a multicentre early experience in Japan. Eur J Cardiothorac Surg 2016;49:1270-8. [Crossref] [PubMed]
- Shrestha M, Pichlmaier M, Martens A, et al. Total aortic arch replacement with a novel four-branched frozen elephant trunk graft: first-in-man results. Eur J Cardiothorac Surg 2013;43:406-10. [Crossref] [PubMed]
- Jakob H, Tsagakis K, Szabo A, et al. Rapid and safe direct cannulation of the true lumen of the ascending aorta in acute type A aortic dissection. J Thorac Cardiovasc Surg 2007;134:244-5. [Crossref] [PubMed]
- Tsagakis K, Kamler M, Benedik J, et al. Angioscopy--a valuable tool in guiding hybrid stent grafting and decision making during type A aortic dissection surgery. Eur J Cardiothorac Surg 2010;38:507-9. [Crossref] [PubMed]
- Tsagakis K, Benedik J, El Khoury G, et al. Aortic valve repair: Intraoperative evaluation of valve geometry by angioscopy. J Thorac Cardiovasc Surg 2015;149:1666-8. [Crossref] [PubMed]
- Leontyev S, Tsagakis K, Pacini D, et al. Impact of Clinical Factors and Surgical Techniques on Early Outcome of Patients Treated With Frozen Elephant Trunk Technique by Using EVITA Open Stent-Graft: Results of a Multicentre Study. Eur J Cardiothorac Surg 2016;49:660-6. [Crossref] [PubMed]
- Jakob H, Dohle DS, Piotrowski J, et al. Six-year Experience With a Hybrid Stent Graft Prosthesis for Extensive Thoracic Aortic Disease: An Interim Balance. Eur J Cardiothorac Surg 2012;42:1018-25. [Crossref] [PubMed]
- Tsagakis K, Dohle DS, Wendt D, et al. Left subclavian artery rerouting and selective perfusion management in frozen elephant trunk surgery. Minim Invasive Ther Allied Technol 2015;24:311-6. [PubMed]
- Harky A, Fok M, Bashir M. Which is the Optimal Frozen Elephant Trunk? A Systematic Review and Meta-Analysis of Outcomes in 2161 Patients Undergoing Thoracic Aortic Aneurysm Surgery Using E-vita OPEN PLUS Hybrid Stent Graft versus Thoraflex™ Hybrid Prosthesis. Brazilian Journal of Cardiovascular Surgery 2020. [Crossref]
- Tsagakis K, Dohle DS, Jánosi RA, et al. Abstract 19090: Identification and Classification of Descending Aorta Re-entry Sites in Type I Aortic Dissection. Circulation 2015;132:A19090.
- Tsagakis K, Jakob H. Which Frozen Elephant Trunk Offers the Optimal Solution? Reflections From Essen Group. Semin Thorac Cardiovasc Surg 2019;31:679-85. [Crossref] [PubMed]
- Jakob H, Shehada S, Dohle DS, et al. New 3 Zones Hybrid Graft: First in Man Experience in Acute Type I Dissection: accepted for publication. J Thorac Cardiovasc Surg 2020. [Crossref]