Open aortic arch reconstruction
Introduction
Aortic arch reconstruction remains a formidable operative procedure. Pioneering work by DeBakey et al. in 1957 identified neurological complications as a predominant cause of high mortality rate (1). The introduction of deep hypothermic circulatory arrest (HCA) by Griepp et al. simplified the operative approach and established a safe strategy for neuroprotection (2). Seminal work by Crawford and associates demonstrated that these techniques could result in death and stroke rates of 10% and 7% respectively (3). Given the emerging role of thoracic aortic endovascular repair (TEVAR) and its encroachment into the arch segment, we initiated an evaluation of open arch reconstruction to identify the important risk factors for poor outcomes, and define its late results (4).
Methods
This study was approved by the Institutional Review Board (IRB) of the University of Michigan Hospitals (IRB study #2003-0128).
A retrospective analysis of data from all patients admitted to the University of Michigan Hospitals from 1993 to 2009 who underwent aortic arch replacement via a median sternotomy was performed (n=721). Details of the operative technique have been described in our previous work (5).
Statistical methods
The primary late outcome of interest was vital status. Follow-up was 100% complete (mean 52.6±39.9 months). Statistical analysis was performed using SPSS (SPSS Inc., Chicago, IL,USA). All data are expressed as mean ± standard deviation where applicable. Univariate analysis was performed with chi-square analysis and independent t-tests. Multivariate models were constructed to identify factors that were independently associated with each of the outcomes of interest. Survival was analyzed by Kaplan-Meier methods. All results with P<0.05 were considered statistically significant.
Results
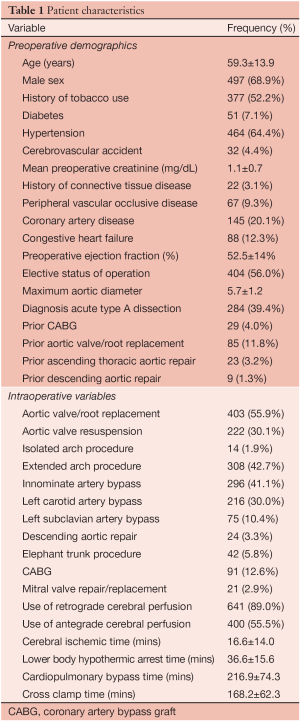
Demographics and comorbidities are listed in Table 1. The procedure was elective in 404 (56%), urgent in 128 (17.8%) and emergent in 188 (26.1%).
Full table
Early results
Early mortality was seen in 36 patients (5.0%). By multivariate analysis, older age (P=0.001; OR, 1.07; 95% CI, 1.0-1.2), lower ejection fraction (P=0.02; OR, 0.97; 95% CI, 0.95-0.99), prolonged cardiopulmonary bypass (P<0.0001; OR, 1.01; 95% CI, 1.007-1.02) and hypothermic circulatory arrest time (P=0.02; OR, 1.03; 95% CI, 1.004-1.05) were independently associated with early mortality. Stroke was identified in 34 patients (4.7%). By multivariate analysis, independent predictors of stroke included history of COPD (P=0.01; OR, 3.3; 95% CI, 1.3-8.2), procedure for type A dissection (P=0.003; OR, 4.05; 95% CI, 1.6-10.1), prolonged HCA time (P=0.03; OR, 1.02; 95% CI, 1.002-1.04), resection into proximal descending aorta (P=0.03; OR, 4.63; 95% CI, 1.12-19.1), and occurrence of permanent postoperative dialysis (P=0.003; OR, 7.14; 95% CI, 2.0-26.1). Finally, other early adverse events included the occurrence of postoperative renal failure in 42 patients (5.8%), with permanent dialysis required in only 14 (1.9%).
Late results
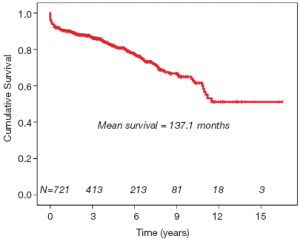
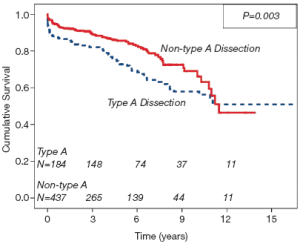
The crude mortality rate at last follow-up was 21.6% (n=156). The Kaplan-Meier survival curve generated for the entire cohort is demonstrated in Figure 1. Independent predictors of late mortality identified through Cox proportional hazards analysis include, increasing age (P<0.0001, HR=1.05), preoperative creatinine (P=0.005, HR=1.57), prior history of CABG (P<0.0001, HR=5.62), descending aortic replacement (P=0.001, HR=11.14), and prolonged circulatory arrest time (P=0.008, HR=1.021), as well as requirement for postoperative tracheostomy (P=0.032, HR=3.16). Although not identified as an independent risk factor on multivariate analysis, type A dissection did have an important time-dependent effect on mortality, particularly during the early postoperative period (Figure 2). Freedom from late aortic reoperation at 10 years was 72.6%.
Discussion
The data in this study suggest that open arch reconstruction can be performed safely. Early results indicate that the dreaded complications of arch repair, namely death and stroke, can occur at rates under 5%. The rates of these complications in our study are comparable to those reported in the contemporary era, despite a high frequency of acute type A dissection (39.3%), reoperative procedures (15.4%), and extended arch repair (42.7%) (6-10). Late results suggest important risk factors for death include age, impaired renal function and a prior history of CABG.
While TEVAR was not an option for the majority of patients in this study, it is important to note that certain groups emerge as ideal candidates for application of this newer technology for the arch aorta. These include older patients, those with renal failure, and those with acute type A dissection. As the Stanford group has suggested in their analysis of patients presenting with bicuspid aortic valve and ascending aneurysms, most patients will have enlargement in the root and proximal ascending aorta, where the complex anatomy of the sinuses, sinotubular junction and the coronary ostia make TEVAR unfeasible with current technology (11). Their work suggests that in identifying an endovascular solution to the ascending aorta, a valved conduit that addresses the coronary arteries may need to be considered as an option.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- De Bakey ME, Crawford ES, Cooley DA, et al. Successful resection of fusiform aneurysm of aortic arch with replacement by homograft. Surg Gynecol Obstet 1957;105:657-64.
- Griepp RB, Stinson EB, Hollingsworth JF, et al. Prosthetic replacement of the aortic arch. J Thorac Cardiovasc Surg 1975;70:1051-63.
- Svensson LG, Crawford ES, Hess KR, et al. Deep hypothermia with circulatory arrest. Determinants of stroke and early mortality in 656 patients. J Thorac Cardiovasc Surg 1993;106:19-28; discussion 28-31.
- Patel HJ, Williams DM, Upchurch GR Jr, et al. Long-term results from a 12-year experience with endovascular therapy for thoracic aortic disease. Ann Thorac Surg 2006;82:2147-53.
- Patel HJ, Nguyen C, Diener AC, et al. Open arch reconstruction in the endovascular era: analysis of 721 patients over 17 years. J Thorac Cardiovasc Surg 2011;141:1417-23.
- Estrera AL, Miller CC 3rd, Lee TY, et al. Ascending and transverse aortic arch repair: the impact of retrograde cerebral perfusion. Circulation 2008;118:S160-6.
- Okita Y, Minatoya K, Tagusari O, et al. Prospective comparative study of brain protection in total aortic arch replacement: deep hypothermic circulatory arrest with retrograde cerebral perfusion or selective antegrade cerebral perfusion. Ann Thorac Surg 2001;72:72-9.
- Milewski RK, Pacini D, Moser GW, et al. Retrograde and antegrade cerebral perfusion: results in short elective arch reconstructive times. Ann Thorac Surg 2010;89:1448-57.
- Halkos ME, Kerendi F, Myung R, et al. Selective antegrade cerebral perfusion via right axillary artery cannulation reduces morbidity and mortality after proximal aortic surgery. J Thorac Cardiovasc Surg 2009;138:1081-9.
- Iba Y, Minatoya K, Matsuda H, et al. Contemporary open aortic arch repair with selective cerebral perfusion in the era of endovascular aortic repair. J Thorac Cardiovasc Surg 2013;145:S72-7.
- Fazel SS, Mallidi HR, Lee RS, et al. The aortopathy of bicuspid aortic valve disease has distinctive patterns and usually involves the transverse aortic arch. J Thorac Cardiovasc Surg 2008;135:901-7, 907.e1-2.





