Degree of hypothermia in aortic arch surgery – optimal temperature for cerebral and spinal protection: deep hypothermia remains the gold standard in the absence of randomized data
Since the pioneering report by Griepp in 1975, the use of deep hypothermia for cerebral and visceral organ protection has ushered in the modern era of safe and effective operation on the aortic arch during circulatory arrest (1). In large part due to advanced circulatory management strategies, the number of proximal aorta and arch replacements have increased each year since 2005, with over 10,000 operations reported to the Society of Thoracic Surgeons National Database in 2009 alone (2). However, despite these advances, neurologic complications remain a sobering limitation of arch repair, with national rates of major neurologic morbidity ranging between 3-5% for elective arch repairs and 9-13% for non-elective repairs (2). In addition, visceral organ injury such as renal failure requiring dialysis occurs in 3-6% of patients (2), highlighting the need for additional investigation into methods for ischemic end-organ protection during circulatory arrest.
The concept of using hypothermia to temporarily reduce the oxygen and metabolic requirements of hypoxic tissues is intuitive and supported by decades of laboratory, translational, and clinical science. Nonetheless, the optimal temperature for hypothermic circulatory arrest (HCA) during arch surgery remains unclear and is confounded by a myriad of other clinical variables that are also without consensus, such as location of temperature measurement, cannulation site, perfusion rates, rapidity of cooling and rewarming, anesthetic and pharmacologic adjuncts, selective cerebral perfusion technique, and use of intraoperative electroencephalographic (EEG) neuromonitoring to guide cooling. As a result, HCA strategies vary considerably, even between respected high-volume centers, and are often dictated as much by dogma and tradition as by evidence.
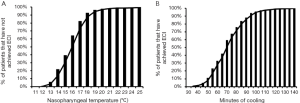
Maximal suppression of the cerebral metabolic rate of oxygen consumption occurs at EEG isoelectricity, or electrocerebral inactivity (ECI) (3,4), with modern aortic surgeons traditionally aiming to achieve this level of metabolic suppression through the use of ‘profound’ or ‘deep’ hypothermia. Although cooling below 18 °C was initially thought necessary to achieve ECI (3,4), more recent studies have shown that ECI can occur anywhere between 10 and 27 °C in human subjects (Figure 1) (5-7). As a result, many experienced centers, including our own, employ neurophysiologic intraoperative monitoring with EEG to precisely detect ECI prior to the initiation of circulatory arrest (6,8-10). Cooling to ECI by EEG ensures maximal suppression of cerebral metabolic activity prior to circulatory arrest, while minimizing perfusion time and hypothermic injury by avoiding excessive cooling (5).
However, as early as 1983, concerns over the physiologic consequences of profound temperature reductions led some to advocate lesser degrees of hypothermia with circulatory arrest (11). Initial concerns focused primarily on bleeding complications thought to result from hypothermia-induced coagulopathy, as well as increased systemic inflammatory response from the prolonged perfusion times required for cooling and rewarming (11,12). More recently, concerns over subtle neurocognitive deficits caused by hypothermic neuronal injury have been raised (13-15), despite reports documenting complete neurocognitive preservation following deep HCA (16). In light of these theoretic concerns along with the advent of cerebral protection strategies, an increasing number of centers now employ ‘moderate’ or even ‘mild’ degrees of systemic hypothermia coupled with selective antegrade cerebral perfusion (SACP) (12,17-21). Although the benefits of SACP with HCA appear well established (10), there remains a lack of objective data demonstrating the superiority, or even non-inferiority, of moderate hypothermia with SACP in comparison to deep hypothermia with SACP, particularly pertaining to visceral organ and spinal cord protection (9).
In the present article, we provide a brief overview of the history and evidence that shapes the current controversy regarding the optimal temperature for central nervous system and visceral organ protection with HCA employed during aortic arch repair. We conclude that, given the limitations of existing retrospective observational data, a multi-center randomized trial is needed to directly compare deep and moderate HCA and provide high-quality evidence-based guidelines for this critically important component of aortic arch repair.
Early history
Early canine experiments with deep HCA were performed by Bigelow and colleagues at the University of Toronto in the 1940s and 1950s. Deep hypothermia was initially tolerated down to 15-20 °C, and allowed isolation of the heart for up to 15 minutes while surgery was performed (22,23). With metabolic activity measured at 15% of that at normothermia, deep HCA appeared a promising technique for cardiac surgery. Further success using hypothermia in neonates and infants in the early 1970s laid crucial groundwork for the later use of deep HCA in aortic arch surgery (24). In 1975, Griepp and colleagues at Stanford University reported four cases of prosthetic aortic arch replacement using deep HCA (1). The patients were cooled to an average esophageal temperature of 14 °C and sustained an average duration of 43 minutes of cerebral ischemia. Three of the four patients survived to hospital discharge, and this report led to the widespread adoption of deep HCA techniques with aortic arch surgery.
Temperature selection in animal models
Multiple studies using animal models that have attempted to shed light on the optimal temperature for HCA have generally supported the use of deeper levels of hypothermia. In a canine model, Mezrow and colleagues compared 60 minutes of HCA at 8, 13, and 18 °C and found that cerebral oxygen consumption remained elevated at 34% of baseline in the 18 °C group, with persistent slow wave activity observed on EEG. However, cerebral oxygen consumption was reduced to 5% and 8% of baseline in the 8 and 13 °C groups and EEG silence was uniformly achieved, suggesting HCA at 8 or 13 °C may provide superior cerebral protection than 18 °C (4). Similarly, Ananiadou and colleagues compared 75 minutes of HCA at 10 and 18 °C in a porcine model and found that cooling to 10 °C led to reduced neuronal injury on histologic examination (25). Strauch and associates randomized pigs to 30 minutes of HCA at 20 °C followed by 60 minutes of HCA with selective cerebral perfusion at 10, 15, 20, or 25 °C, and reported decreased oxygen consumption and improved postoperative behavioral scores with selective cerebral perfusion at 10 to 15 °C (26). Beyond decreased metabolic demand, rat models have demonstrated that small ubiquitin-like modifier (SUMO) conjugation of target proteins is markedly activated in the brain during deep (18 °C) to moderate (24 °C) hypothermia compared to milder temperatures (30 and 37 °C), resulting in an increased tolerance to stress as another mechanism of neuroprotection (27).
In addition to cerebral protection, canine, porcine, and rat models have been used to assess hypothermia for protection of other organ systems. Spinal cord hypothermia at 13 to 18 °C in a distal aortic cross-clamp model protected 100% (9 of 9) of dogs from paraplegia compared to 0% (0 of 8) of dogs with normothermic spinal cord protection (28). Likewise, pigs tolerated only 30 minutes of aortic cross-clamp with normothermic spinal protection compared to 50 minutes with mild hypothermia (32 °C) (29). In a circulatory arrest model, evaluation of moderate hypothermia (28 °C) in pigs revealed an alarmingly high incidence of irreversible paraplegia and multi-system organ failure at 90 minutes (30). Finally, moderate hypothermia (30 to 32 °C) compared to normothermia in rats and deep hypothermia (20 °C) compared to moderate hypothermia (30 °C) in pigs both demonstrated reduced oxidative and ischemic stress in visceral organs such as the kidneys and small intestines for cooler temperatures during circulatory arrest (31,32).
Human physiology
Several studies examining the physiology of deep HCA have validated the principles of the technique in humans. In a study of 37 adults undergoing deep HCA, cerebral metabolic rates of 17% of baseline were achieved at 15 °C, suggesting deep hypothermia provides a 6-fold increase in the safe duration of circulatory arrest at 15 °C compared to 5 minutes at normothermia (33). In a study of 47 adults undergoing HCA with EEG monitoring, Stecker and colleagues elegantly demonstrated that ECI, and thus maximal suppression of cerebral oxygen metabolism, was achieved over a range of temperatures between 12.5 and 27.2 °C, with a mean temperature at ECI of 17.3 °C (7). Our group recently validated these results in a larger cohort of 325 patients undergoing deep HCA with EEG monitoring and also found that patient-specific factors were poor predictors of the temperature or cooling time required to achieve ECI, therefore necessitating intra-operative EEG monitoring for precise ECI detection (5).
Outcomes with deep hypothermia
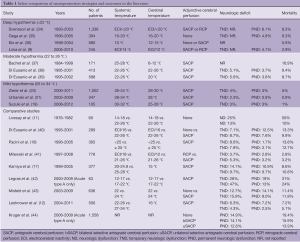
Recent single-institution reports from experienced aortic centers have established benchmark outcomes using refined deep HCA techniques (Table 1). In a series of deep HCA without cerebral perfusion, Elefteriades reported 394 cases (22.1% non-elective) where bladder temperatures were taken to 16-20 °C with no EEG monitoring. Mean circulatory arrest time was 31 minutes and ranged from 10 to 66 minutes. Mortality and stroke rates were 6.3% and 4.8%, respectively (35). In 2004, Svensson reported a series of 1,352 patients undergoing proximal aorta, arch, and/or descending aorta repairs with deep HCA and selective ACP or RCP, of which 20% of operations were emergent and 32% were reoperative. Patients were cooled to ECI when EEG was available or to a minimum temperature of 20 °C. Postoperative rates of stroke and operative mortality were 6.1% and 8.3% (34). In 2008, Griepp reported a series of 680 root/ascending aorta and/or transverse arch repairs performed using deep HCA at 12 to 15 °C with SACP at 15 to 20 °C. This series included elective and non-elective cases as well as 29% reoperative patients, with overall operative mortality of 5.9% (36). In 2010, Bavaria reported a series of 682 patients who underwent non-emergent proximal aortic reconstruction with deep HCA and retrograde cerebral perfusion (RCP) at 10 to 12 °C, of which 25% of cases were reoperative. Patients were cooled to ECI when EEG was available or for a minimum cooling time of 45 minutes. Postoperative rates of permanent neurologic deficit, reoperation for bleeding, and operative mortality were 2.8%, 3.8%, and 2.8%, respectively (41). In 2011, we published our results with deep HCA and selective cerebral perfusion in a series of 245 hemi-arch replacements, of which 36% of operations were urgent or emergent and 16% were reoperations (9). Patients were cooled to ECI when EEG was available, or for a minimum cooling time of 50 minutes, or until the nasopharyngeal temperature was below 18 °C. SACP (89% of patients) or RCP (11% of patients) was maintained at 12 °C. Postoperative rates of stroke, re-exploration for bleeding, new onset dialysis, and operative mortality were 4.1%, 2.9%, 1.2%, and 2.9%, respectively. The rate of stroke in elective cases was 0.8%. Collectively, these results demonstrate that excellent outcomes can be achieved with deep HCA at expert centers treating diverse patients with complex aortic pathologies.
Full table
Comparisons with lesser degrees of hypothermia
Since the introduction of retrograde (45) and antegrade (46) selective cerebral perfusion strategies in the mid-1980s, some have called into question the continued need for deep systemic hypothermia with circulatory arrest. In addition, these techniques have increased the complexity of optimal temperature analyses, as lower body hypothermia and cerebral hypothermia can be separate variables, and the type of cerebral perfusion strategy becomes a significant confounder. While several randomized controlled trials (RCTs) have examined or are currently examining the question of hypothermia in coronary artery bypass surgery (47,48) and valve surgery (49,50), there are no completed or enrolling studies examining the optimal degree of hypothermia during circulatory arrest for aortic arch surgery.
Retrospective single-institution studies reporting outcomes for a single technique are numerous in the literature and demonstrate the variability in current practice. Acceptable outcomes have been reported from centers using moderate (22 to 26 °C) (21,38) or mild (25 to 34 °C) (19-21) systemic and cerebral hypothermia (Table 1). However, these studies lack control groups, and comparisons to other studies are limited given differences in repairs and patient populations.
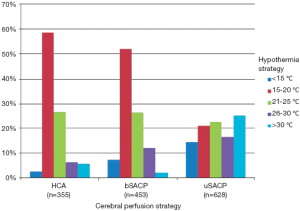
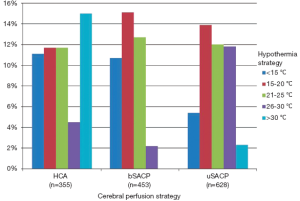
Although RCTs are lacking, several observational studies have attempted to address neuroprotection with deep hypothermia in adjusted analyses. In a retrospective, propensity-matched analysis of 220 patients undergoing deep HCA versus those undergoing normothermic cardiopulmonary bypass during ascending and/or arch replacement, Kunihara and colleagues found HCA was not associated with postoperative neurologic dysfunction or increased mortality (51). A study from France comparing deep hypothermia (<17 °C) to moderate hypothermia (≥17 °C) during emergent type A dissection repair found no difference in neurologic deficits between groups, but did note decreased infection rates and intensive care unit stay with deep hypothermia (42). Finally, a study of the German Registry for Acute Aortic Dissection Type A (GERAADA) showed divergent practice patterns of hypothermia and cerebral perfusion use for acute type A dissection repair (44). Out of 1,558 patients in the registry, 94% were managed with HCA and 6% were managed with cardiopulmonary bypass alone. Of the 1,470 HCA patients, 22.8% received no selective cerebral perfusion, 2.2% received retrograde cerebral perfusion, 29.1% received bilateral selective antegrade cerebral perfusion, and 40.3% received unilateral selective antegrade cerebral perfusion. The temperatures used with each HCA technique further varied between <15 °C and >30 °C (Figure 2). Despite a large sample size, no statistically significant differences could be found between the rate of permanent neurologic dysfunction and the degree of cerebral hypothermia and/or cerebral perfusion strategy utilized (Figure 3), although the study had many major limitations including a lack of risk stratification between groups. The most significant predictor of mortality in the GERAADA study was circulatory arrest time >30 minutes in the non-SACP groups.
Comparative studies supporting more moderate degrees of hypothermia have been intriguing, but have generally failed to contain suitable control groups of patients undergoing deep hypothermia (Table 1). In a retrospective, propensity-matched analysis comparing systemic deep HCA (20 to 24.9 °C) to moderate HCA (25 to 28 °C) in 180 aortic arch surgery patients, Kamiya and colleagues found no difference in neurologic dysfunction between the two groups (17). The rate of re-exploration for bleeding was also not reduced in the moderate hypothermia group. The incidence of paralysis was too low to make any significant conclusions, although the authors did suggest a trend of decreased risk of paralysis with deep versus moderate hypothermia for patients undergoing circulatory arrest for >60 minutes. No conclusions could be drawn about cerebral temperature as all patients underwent SACP with perfusate at 15 °C. Finally, the temperatures used in the deep HCA group (20 to 24.9 °C) would be considered moderate by most practitioners and do not represent a true comparison to deep hypothermia.
In a retrospective comparison of deep HCA alone (<20 °C) to moderate HCA (22 to 26 °C) with SACP, Di Eusanio and colleagues found no differences in postoperative neurologic dysfunction, but did note increased mechanical ventilation time by 1.5 days in the deep HCA group (40). Here, the lack of selective cerebral perfusion in the deep HCA group confounded the comparison of hypothermic temperatures. Finally, a study examining patients undergoing HCA with core temperatures ≥25 °C versus <25 °C showed no differences in neurologic dysfunction (18). Again, the patients in the cold group were not cooled to sufficient temperatures to be considered representative of a deep HCA technique. Although these retrospective observational studies are insufficient to prove superiority or non-inferiority of one approach compared to another, they do appear to establish the necessary equipoise required to justify a RCT in this area.
Future directions
There are several limitations to the current study of optimal temperature for circulatory arrest during aortic arch surgery. The lack of high-quality evidence in the current literature and dramatic variability between practitioners complicates the development of a standard study protocol. A uniform set of variables needs to be established and reported in analyses comparing neuroprotection strategies for circulatory arrest in aortic arch surgery. These variables may include cerebral temperature, systemic temperature, method of temperature assessment, cerebral perfusion strategy (none, RCP, unilateral SACP, bilateral SACP), perfusion pressure, perfusate type and temperature, circulatory arrest time, complexity of arch repair, along with more conventional patient and operative characteristics.
There is a further lack of standard terminology regarding definitions of mild, moderate, and deep hypothermia. Evaluating studies that claim to use a protocol with a specific degree of hypothermia becomes difficult when groups reporting moderate hypothermia use temperature ranges that other groups would consider as deep or mild hypothermia. Additionally, it becomes problematic to compare studies with protocols that use ranges which cross the deep-moderate or moderate-mild border. Ideally, standard definitions of mild, moderate, and deep hypothermia would be established, and all studies would report the exact temperature ranges used.
In addition, reported outcomes from studies examining varying degrees of HCA need to be expanded. While consistent definitions of mortality and neurologic dysfunction are widespread (52), more sensitive measures of neurocognitive function are needed to better assess the effects of various temperatures on the brain. In addition to neurologic effects, systemic and visceral organ outcomes need to be evaluated and reported. Temperatures utilized for HCA may affect ischemic end-organ protection, coagulation, circulatory and inflammatory response systems, leading to differences in bleeding, embolic events, renal perfusion, and hepatic function, among others. Studies evaluating HCA will also need to be stratified by procedure type (i.e. hemi-arch vs. total arch replacement), as temperatures that are safe for shorter procedures may not be tolerated with more complex procedures requiring longer HCA times.
The variability in current practice and the paucity of adequately controlled comparative studies demonstrate the need for a RCT to address this issue. Significantly, the disparate results from several smaller studies indicate that the difference in outcomes between circulation strategies may be small, therefore requiring a large sample size to adequately evaluate. Alternatively, a large, rigorous, adjusted analysis would also be a welcome addition to the literature and as a guide to therapy. However, any dataset used to perform this analysis would require robust data collection to include many of the esoteric variables unique to arch surgery described above.
Conclusions
The optimal degree of hypothermia for circulatory arrest in aortic arch surgery has been vigorously debated since the technique gained popularity in the 1970s. Significant improvements have been made over the last 35 years, allowing for increased operative times and for more complex procedures to be performed. However, postoperative neurologic morbidity and mortality remain elevated. The complexity of the procedures and the difficulties in objectively comparing perfusion techniques necessitate a standardized approach to the analysis and presentation of research in this field. With a number of potential variables under the control of the surgical team, including degree of hypothermia and cerebral perfusion strategy, robust studies are needed to determine the optimal approach to these patients in order to further decrease postoperative complications. However, until definitive comparative studies have been completed, we suggest deep hypothermia, as established and optimized by nearly four decades of preclinical and clinical data, remains the gold standard for end-organ protection with circulatory arrest during aortic arch repair.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Griepp RB, Stinson EB, Hollingsworth JF, et al. Prosthetic replacement of the aortic arch. J Thorac Cardiovasc Surg 1975;70:1051-63.
- Williams JB, Peterson ED, Zhao Y, et al. Contemporary results for proximal aortic replacement in North America. J Am Coll Cardiol 2012;60:1156-62.
- Michenfelder JD, Milde JH. The effect of profound levels of hypothermia (below 14 degrees C) on canine cerebral metabolism. J Cereb Blood Flow Metab 1992;12:877-80.
- Mezrow CK, Midulla PS, Sadeghi AM, et al. Evaluation of cerebral metabolism and quantitative electroencephalography after hypothermic circulatory arrest and low-flow cardiopulmonary bypass at different temperatures. J Thorac Cardiovasc Surg 1994;107:1006-19.
- James ML, Andersen ND, Swaminathan M, et al. Predictors of Electrocerebral Inactivity with Deep Hypothermia. American Association for Thoracic Surgery Aortic Symposium 2012, April 26-27, New York.
- Coselli JS, Crawford ES, Beall AC Jr, et al. Determination of brain temperatures for safe circulatory arrest during cardiovascular operation. Ann Thorac Surg 1988;45:638-42.
- Stecker MM, Cheung AT, Pochettino A, et al. Deep hypothermic circulatory arrest: I. Effects of cooling on electroencephalogram and evoked potentials. Ann Thorac Surg 2001;71:14-21.
- Bavaria JE, Pochettino A, Brinster DR, et al. New paradigms and improved results for the surgical treatment of acute type A dissection. Ann Surg 2001;234:336-42; discussion 342-3.
- Lima B, Williams JB, Bhattacharya SD, et al. Results of proximal arch replacement using deep hypothermia for circulatory arrest: is moderate hypothermia really justifiable? The American surgeon 2011;77:1438-44.
- Svensson LG. Surgery of the Aortic Arch. In: Sellke FW, Del Nido PJ, Swanson SJ. eds. Surgery of the Chest. Vol II. 8th ed. Philadelphia, PA: Saunders Elsevier, 2010.
- Livesay JJ, Cooley DA, Reul GJ, et al. Resection of aortic arch aneurysms: a comparison of hypothermic techniques in 60 patients. Ann Thorac Surg 1983;36:19-28.
- Leshnower BG, Myung RJ, Thourani VH, et al. Hemiarch replacement at 28°C: an analysis of mild and moderate hypothermia in 500 patients. Ann Thorac Surg 2012;93:1910-5; discussion 1915-6.
- Kumral E, Yuksel M, Buket S, et al. Neurologic complications after deep hypothermic circulatory arrest: types, predictors, and timing. Tex Heart Inst J 2001;28:83-8.
- Reich DL, Uysal S, Sliwinski M, et al. Neuropsychologic outcome after deep hypothermic circulatory arrest in adults. J Thorac Cardiovasc Surg 1999;117:156-63.
- Welz A, Pogarell O, Tatsch K, et al. Surgery of the thoracic aorta using deep hypothermic total circulatory arrest. Are there neurological consequences other than frank cerebral defects? Eur J Cardiothorac Surg 1997;11:650-6.
- Percy A, Widman S, Rizzo JA, et al. Deep hypothermic circulatory arrest in patients with high cognitive needs: full preservation of cognitive abilities. Ann Thorac Surg 2009;87:117-23.
- Kamiya H, Hagl C, Kropivnitskaya I, et al. The safety of moderate hypothermic lower body circulatory arrest with selective cerebral perfusion: a propensity score analysis. J Thorac Cardiovasc Surg 2007;133:501-9.
- Pacini D, Leone A, Di Marco L, et al. Antegrade selective cerebral perfusion in thoracic aorta surgery: safety of moderate hypothermia. Eur J Cardiothorac Surg 2007;31:618-22.
- Suzuki T, Asai T, Nota H, et al. Selective cerebral perfusion with mild hypothermic lower body circulatory arrest is safe for aortic arch surgery. Eur J Cardiothorac Surg 2013. [Epub ahead of print].
- Zierer A, El-Sayed Ahmad A, Papadopoulos N, et al. Selective antegrade cerebral perfusion and mild (28 degrees C-30 degrees C) systemic hypothermic circulatory arrest for aortic arch replacement: results from 1002 patients. J Thorac Cardiovasc Surg 2012;144:1042-9.
- Urbanski PP, Lenos A, Bougioukakis P, et al. Mild-to-moderate hypothermia in aortic arch surgery using circulatory arrest: a change of paradigm? Eur J Cardiothorac Surg 2012;41:185-91.
- Bigelow WG, Callaghan JC, Hopps JA. General hypothermia for experimental intracardiac surgery; the use of electrophrenic respirations, an artificial pacemaker for cardiac standstill and radio-frequency rewarming in general hypothermia. Ann Surg 1950;132:531-9.
- Bigelow WG, Lindsay WK, Greenwood WF. Hypothermia; its possible role in cardiac surgery: an investigation of factors governing survival in dogs at low body temperatures. Ann Surg 1950;132:849-66.
- Barratt-Boyes BG, Simpson M, Neutze JM. Intracardiac surgery in neonates and infants using deep hypothermia with surface cooling and limited cardiopulmonary bypass. Circulation 1971;43:I25-30.
- Ananiadou OG, Bibou K, Drossos GE, et al. Hypothermia at 10 degrees C reduces neurologic injury after hypothermic circulatory arrest in the pig. J Card Surg 2008;23:31-8.
- Strauch JT, Spielvogel D, Lauten A, et al. Optimal temperature for selective cerebral perfusion. J Thorac Cardiovasc Surg 2005;130:74-82.
- Wang L, Ma Q, Yang W, et al. Moderate hypothermia induces marked increase in levels and nuclear accumulation of SUMO2/3-conjugated proteins in neurons. J Neurochem 2012;123:349-59.
- Berguer R, Porto J, Fedoronko B, et al. Selective deep hypothermia of the spinal cord prevents paraplegia after aortic cross-clamping in the dog model. J Vasc Surg 1992;15:62-71; discussion 71-2.
- Strauch JT, Lauten A, Spielvogel D, et al. Mild hypothermia protects the spinal cord from ischemic injury in a chronic porcine model. Eur J Cardiothorac Surg 2004;25:708-15.
- Etz CD, Luehr M, Kari FA, et al. Selective cerebral perfusion at 28 degrees C--is the spinal cord safe? Eur J Cardiothorac Surg 2009;36:946-55.
- Stefanutti G, Pierro A, Vinardi S, et al. Moderate hypothermia protects against systemic oxidative stress in a rat model of intestinal ischemia and reperfusion injury. Shock 2005;24:159-64.
- Khaladj N, Peterss S, Pichlmaier M, et al. The impact of deep and moderate body temperatures on end-organ function during hypothermic circulatory arrest. Eur J Cardiothorac Surg 2011;40:1492-9; discussion 1499.
- McCullough JN, Zhang N, Reich DL, et al. Cerebral metabolic suppression during hypothermic circulatory arrest in humans. Ann Thorac Surg 1999;67:1895-9; discussion 1919-21.
- Svensson LG, Blackstone EH, Rajeswaran J, et al. Does the arterial cannulation site for circulatory arrest influence stroke risk? Ann Thorac Surg 2004;78:1274-84; discussion 1274-84.
- Gega A, Rizzo JA, Johnson MH, et al. Straight deep hypothermic arrest: experience in 394 patients supports its effectiveness as a sole means of brain preservation. Ann Thorac Surg 2007;84:759-66; discussion 766-7.
- Etz CD, Plestis KA, Homann TM, et al. Reoperative aortic root and transverse arch procedures: a comparison with contemporaneous primary operations. J Thorac Cardiovasc Surg 2008;136:860-7, 867.e1-3.
- Bachet J, Guilmet D, Goudot B, et al. Antegrade cerebral perfusion with cold blood: a 13-year experience. Ann Thorac Surg 1999;67:1874-8; discussion 1891-4.
- Di Eusanio M, Schepens MA, Morshuis WJ, et al. Antegrade selective cerebral perfusion during operations on the thoracic aorta: factors influencing survival and neurologic outcome in 413 patients. J Thorac Cardiovasc Surg 2002;124:1080-6.
- Di Eusanio M, Schepens MA, Morshuis WJ, et al. Brain protection using antegrade selective cerebral perfusion: a multicenter study. Ann Thorac Surg 2003;76:1181-8; discussion 1188-9.
- Di Eusanio M, Wesselink RM, Morshuis WJ, et al. Deep hypothermic circulatory arrest and antegrade selective cerebral perfusion during ascending aorta-hemiarch replacement: a retrospective comparative study. J Thorac Cardiovasc Surg 2003;125:849-54.
- Milewski RK, Pacini D, Moser GW, et al. Retrograde and antegrade cerebral perfusion: results in short elective arch reconstructive times. Ann Thorac Surg 2010;89:1448-57.
- Legras A, Bruzzi M, Nakashima K, et al. Colder is better during hypothermic circulatory arrest for acute type an aortic dissection. Scand Cardiovasc J 2013;47:121-8.
- Misfeld M, Leontyev S, Borger MA, et al. What is the best strategy for brain protection in patients undergoing aortic arch surgery? A single center experience of 636 patients. Ann Thorac Surg 2012;93:1502-8.
- Krüger T, Weigang E, Hoffmann I, et al. Cerebral protection during surgery for acute aortic dissection type A: results of the German Registry for Acute Aortic Dissection Type A (GERAADA). Circulation 2011;124:434-43.
- Ueda Y, Miki S, Kusuhara K, et al. Surgical treatment of aneurysm or dissection involving the ascending aorta and aortic arch, utilizing circulatory arrest and retrograde cerebral perfusion. J Cardiovasc Surg (Torino) 1990;31:553-8.
- Bachet J, Guilmet D, Goudot B, et al. Cold cerebroplegia. A new technique of cerebral protection during operations on the transverse aortic arch. J Thorac Cardiovasc Surg 1991;102:85-93; discussion 93-4.
- Martin TD, Craver JM, Gott JP, et al. Prospective, randomized trial of retrograde warm blood cardioplegia: myocardial benefit and neurologic threat. Ann Thorac Surg 1994;57:298-302; discussion 302-4.
- Randomised trial of normothermic versus hypothermic coronary bypass surgery. The Warm Heart Investigators. Lancet 1994;343:559-63.
- Normothermia Versus Hypothermia for Valvular Surgery Patients 2011. Available online: http://clinicaltrials.gov/ct2/show/NCT01685554?term=NCT01685554&rank=1. Accessed 01/28/2013.
- Normothermic Cardiopulonary Bypass Increases Cerebral Oxygenation During Valve Surgery 2012. Available online: http://clinicaltrials.gov/ct2/show/NCT01338961?term=NCT01338961&rank=1. Accessed 01/28/2013.
- Kunihara T, Grun T, Aicher D, et al. Hypothermic circulatory arrest is not a risk factor for neurologic morbidity in aortic surgery: a propensity score analysis. J Thorac Cardiovasc Surg 2005;130:712-8.
- Ergin MA, Galla JD, Lansman sL, et al. Hypothermic circulatory arrest in operations on the thoracic aorta. Determinants of operative mortality and neurologic outcome. J Thorac Cardiovasc Surg 1994;107:788-97; discussion 797-9.





