Type A aortic dissection: the extent of surgical intervention
Acute DeBakey type I aortic dissection is a life-threatening emergency and requires immediate surgical intervention. For untreated DeBakey type I aortic dissection mortality increases up to 50% in the first 24 hours and 75% within two weeks after the initial event (1). Although surgical techniques and perioperative care have significantly improved during the decades, mortality remains high and is reported between 15% and 30% (2-6).
Bearing in mind that this is a high risk surgical subset, the primary target of an emergency operation for acute DeBakey type I aortic dissection has to be survival of the patient with a low morbidity rate. Various steps have been taken over the last few years to improve the surgical outcome of these complex aortic pathologies. The practice of hypothermic circulatory arrest and open distal anastomosis with visual inspection of the arch vessels was introduced in the 1980s and became routine soon afterward. Cerebral protection techniques were introduced in the 1990s. Ultimately, there is strong evidence that antegrade cerebral perfusion has a beneficial effect on neurological outcome. The combination of moderate hypothermic circulatory arrest with cold selective antegrade cerebral perfusion is an adequate tool for neuroprotection during aortic surgery (7,8).
Principle of the operative strategy
The crucial elements for surgical success in acute DeBakey type I aortic dissection are the excision of the primary entry tear, correction of any aortic valve insufficiency, and in order to correct distal malperfusion, restoration of dominant true lumen flow in the downstream aorta. We consider these steps of the operation mandatory for successful treatment of DeBakey type I aortic dissections.
We are convinced that the inspection of the transverse aortic arch is of utmost importance to identify potential intimal entry and re-entry tears. Therefore, we recommend an open distal anastomosis without cross-clamping the aorta. David and colleagues suggested that avoidance of aortic cross-clamping during cooling and resection of the primary tear improved early and late outcome (9). Not only may clamping induce false lumen pressurization leading to propagation of the dissection and malperfusion, but also there is an elevated risk of inducing additional aortic lesions by the clamp itself.
Several factors influence the decision whether a hemiarch replacement is sufficient or a total arch replacement has to be performed. If the entry tear is located in the ascending aorta or in the concavity of the aortic arch, a hemiarch replacement offers a safe therapeutic option, especially in the elderly or unstable patient. Entry tears near the origin of the supra-aortic branches call for total arch replacement, which requires re-implantation of the arch vessels. Circumferential dissection around the brachiocephalic vessels may also indicate the need for total arch replacement. Depending on the morphology and location of the intimal tear the re-implantation of the supra-aortic vessels can be done “en-bloc” as an island of aortic wall or by separate re-implantation of the arch branches using a trifurcated graft. These complex procedures are associated with prolonged circulatory arrest times leading to an elevated risk of neurological complications (10). Taking all precautions for neuroprotection (hypothermia, selective antegrade cerebral perfusion, topical cooling of the head, administration of corticosteroids), circulatory arrest periods of up to 40 minutes can be tolerated without running a major risk of neurological complications (11,12). Kazui and colleagues published excellent results for total arch replacement in selected patients with acute DeBakey type I aortic dissection (13). The decision to perform a complete arch repair was based on factors such as location of intimal tears in the aortic arch or descending aorta, compression of the true lumen, compromised arch vessels, or presence of connective tissue disorder. In contrast to this study other authors discovered an increased mortality and morbidity for total arch replacement as compared to ascending/hemiarch replacement. Furthermore, the incidence of late aortic events influencing survival was not reduced by total arch replacement (14,15).
Kim and colleagues evaluated risk factors for descending aortic aneurysmal changes following surgery for acute DeBakey type I aortic dissection. Postoperative aortic aneurysms were observed most commonly in the proximal descending aorta. No statistically relevant difference between complete versus hemiarch replacement was found. The only significant and independent factor predicting aneurysm formation was the luminal diameter of the proximal descending aorta on initial CT scan, suggesting that adjunctive procedures combined with aortic replacement are needed to prevent late aneurysm formation (16).
Although the outcome of surgical repair of acute DeBakey type I aortic dissection has improved over the decades, the long-term prognosis is limited by residual dissection of the descending aorta. Numerous publications evaluated the effect of a patent false lumen on long-term outcomes after successful operation of DeBakey type I aortic dissection. There is certain evidence that a patent false lumen in the downstream aorta increases the risk of late aortic growth and therefore the necessity for secondary aortic interventions in the follow-up period (17-19). False lumen patency is observed up to 70% of conventionally operated patients. Therefore, an operative strategy has to be pursued, which has the ability to promote obliteration of the false lumen in the descending aorta without increasing the risk of the operation. New hybrid procedures, which combine open antegrade stent-grafting with classical aortic arch surgery, seem to offer a feasible and safe therapeutic option, especially for the younger patient population. One promising approach is the so-called frozen elephant trunk (FET) procedure. Tsagakis and colleagues were able to demonstrate the effect of endovascular treatment on false lumen obliteration in the descending aorta. False lumen thrombosis of the thoracic aorta occurred in more than 90% of patients at first follow-up CT examination following a frozen elephant trunk procedure (20,21). Encouraging mid-term results were also published by Ushida and colleagues. The authors reported a significantly improved 5-years survival rate after FET treatment as compared to conventional hemiarch replacement (22).
Compared to conventional hemiarch replacement the FET procedure is technically more demanding and associated with prolonged circulatory arrest times. Therefore, we recommend this treatment for selected patients in which a beneficial effect on long-term prognosis can be assumed. We apply the frozen elephant trunk technique in the younger patient population (<70 years), especially those with an enlarged proximal descending aorta, entry tears in the proximal descending aorta and/or signs of distal malperfusion. However, in the hand of an un-experienced surgeon we recommend a hemiarch replacement in order to avoid exceeding long circulatory arrest times. As previously mentioned, the main target of the operation has to be the survival of the patient with a low morbidity rate.
How we do it
The surgical access is through a complete median sternotomy. In our department we mainly use the right axillary artery for the arterial inflow of the cardiopulmonary bypass. Once the extra-corporal circulation is established the cooling process is initiated. Depending on the extent of the aortic pathology and the expected circulatory arrest time, it is safe to perform the procedure in moderate hypothermia with a core temperature (bladder or rectal) of 25-28 °C. For brain protection we use bilateral selective antegrade cerebral perfusion (10 mL/kg bodyweight). The left subclavian artery is typically blocked with a 6-F Fogarty catheter to prevent the steal phenomenon and back bleeding. After resection of the dissected aortic tissue the decision is taken whether to pursue the operation with a hemiarch, total arch, or frozen elephant trunk repair. In all cases of dissection we suture the intima-media cylinder to the adventitial aortic layer with Teflon felts (sandwich technique). If the decision is made to go for a FET procedure, the hybrid prosthesis is placed into the descending aorta in an antegrade manner through the open aortic arch. It is recommended to use a guide wire for this maneuver to prevent accidental insertion in the false lumen. Additionally, the correct position of the guide-wire can be confirmed by angioscopy. Thereafter the stent graft is deployed in the descending aorta placing the proximal end of the stent graft 2 cm distal to the offspring of the left subclavian artery. Basically, the replacement of the aortic arch can be done with the integrated tubular graft of the hybrid prosthesis or with a separate vascular graft. If anatomically feasible we prefer to maintain a 2-3 cm junction between the left subclavian artery and the descending aorta. The Dacron of the hybrid prosthesis is trimmed to a 1 cm rim and fixed to the wall of the proximal descending aorta with a 4-0 polyprelene running suture. We call this technique the “peninsula repair” (Figures 1,2) (23,24). Once the arch replacement is accomplished, the graft prosthesis is clamped, and full perfusion and rewarming is restarted. In the rewarming period the proximal anastomosis is completed and concomitant procedures can be performed if necessary.
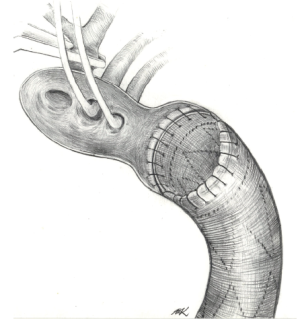
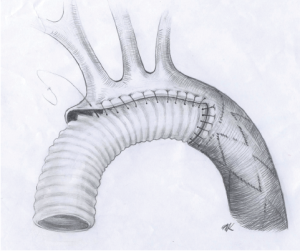
Conclusions
For DeBakey type I dissections the appropriate repair of the aortic arch depends on the location of the primary entry tear and the morphology of the dissection. Primarily, an open distal anastomosis with aggressive hemiarch replacement should be sought. In selected patients a more extensive treatment including the descending aorta seems reasonable with regard to long-term prognosis.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Woo KM, Schneider JI. High-risk chief complaints I: chest pain--the big three. Emerg Med Clin North Am 2009;27:685-712, x.
- Trimarchi S, Nienaber CA, Rampoldi V, et al. Contemporary results of surgery in acute type A aortic dissection: The International Registry of Acute Aortic Dissection experience. J Thorac Cardiovasc Surg 2005;129:112-22.
- Bachet J, Goudot B, Dreyfus GD, et al. Surgery for acute type A aortic dissection: the Hopital Foch experience (1977-1998). Ann Thorac Surg 1999;67:2006-9; discussion 2014-9.
- Heinemann M, Laas J, Jurmann M, et al. Surgery extended into the aortic arch in acute type A dissection. Indications, techniques, and results. Circulation 1991;84:III25-30.
- Fann JI, Smith JA, Miller DC, et al. Surgical management of aortic dissection during a 30-year period. Circulation 1995;92:II113-21.
- Crawford ES, Kirklin JW, Naftel DC, et al. Surgery for acute dissection of ascending aorta. Should the arch be included? J Thorac Cardiovasc Surg 1992;104:46-59.
- Khaladj N, Shrestha M, Meck S, et al. Hypothermic circulatory arrest with selective antegrade cerebral perfusion in ascending aortic and aortic arch surgery: a risk factor analysis for adverse outcome in 501 patients. J Thorac Cardiovasc Surg 2008;135:908-14.
- Krähenbühl ES, Clément M, Reineke D, et al. Antegrade cerebral protection in thoracic aortic surgery: lessons from the past decade. Eur J Cardiothorac Surg 2010;38:46-51.
- David TE, Armstrong S, Ivanov J, et al. Surgery for acute type A aortic dissection. Ann Thorac Surg 1999;67:1999-2001; discussion 2014-9.
- Ehrlich MP, Ergin MA, McCullough JN, et al. Results of immediate surgical treatment of all acute type A dissections. Circulation 2000;102:III248-52.
- Pacini D, Leone A, Di Marco L, et al. Antegrade selective cerebral perfusion in thoracic aorta surgery: safety of moderate hypothermia. Eur J Cardiothorac Surg 2007;31:618-22.
- Di Eusanio M, Schepens MA, Morshuis WJ, et al. Antegrade selective cerebral perfusion during operations on the thoracic aorta: factors influencing survival and neurologic outcome in 413 patients. J Thorac Cardiovasc Surg 2002;124:1080-6.
- Kazui T, Washiyama N, Muhammad BA, et al. Extended total arch replacement for acute type a aortic dissection: experience with seventy patients. J Thorac Cardiovasc Surg 2000;119:558-65.
- Kim JB, Chung CH, Moon DH, et al. Total arch repair versus hemiarch repair in the management of acute DeBakey type I aortic dissection. Eur J Cardiothorac Surg 2011;40:881-7.
- Zierer A, Voeller RK, Hill KE, et al. Aortic enlargement and late reoperation after repair of acute type A aortic dissection. Ann Thorac Surg 2007;84:479-86; discussion 486-7.
- Kim JB, Lee CH, Lee TY, et al. Descending aortic aneurysmal changes following surgery for acute DeBakey type I aortic dissection. Eur J Cardiothorac Surg 2012;42:851-6; discussion 856-7.
- Heinemann M, Laas J, Karck M, et al. Thoracic aortic aneurysms after acute type A aortic dissection: necessity for follow-up. Ann Thorac Surg 1990;49:580-4.
- Ergin MA, Phillips RA, Galla JD, et al. Significance of distal false lumen after type A dissection repair. Ann Thorac Surg 1994;57:820-4; discussion 825.
- Kazui T, Yamashita K, Washiyama N, et al. Impact of an aggressive surgical approach on surgical outcome in type A aortic dissection. Ann Thorac Surg 2002;74:S1844-7; discussion S1857-63.
- Tsagakis K, Pacini D, Di Bartolomeo R, et al. Arch replacement and downstream stent grafting in complex aortic dissection: first results of an international registry. Eur J Cardiothorac Surg 2011;39:87-93; discussion 93-4.
- Gorlitzer M, Weiss G, Meinhart J, et al. Fate of the false lumen after combined surgical and endovascular repair treating Stanford type A aortic dissections. Ann Thorac Surg 2010;89:794-9.
- Uchida N, Shibamura H, Katayama A, et al. Operative strategy for acute type a aortic dissection: ascending aortic or hemiarch versus total arch replacement with frozen elephant trunk. Ann Thorac Surg 2009;87:773-7.
- Gorlitzer M, Weiss G, Thalmann M, et al. Combined surgical and endovascular repair of complex aortic pathologies with a new hybrid prosthesis. Ann Thorac Surg 2007;84:1971-6.
- Weiss G, Gorlitzer M, Folkmann S, et al. Frozen elephant trunk technique for acute type A aortic dissection. Multimedia Man Cardiothoracic Surg 2012; doi:10.1093/mmcts/mms012.





