We should replace the aortic arch and more in DeBakey type I dissection - A perspective from the Cleveland Clinic
Introduction
A frequently debated question in aortic arch surgery has been: should we replace the dissected arch in type A dissection? As techniques improve the answer is shifting towards ‘yes’ more often than ‘no’. Arguments in favor of a more extensive repair for DeBakey type 1 dissection, however, are not new.
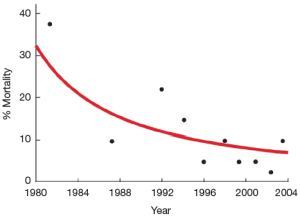
In the early 1980s operative techniques for this disease were refined to greatly reduce operative deaths, especially those attributable to bleeding complications (1, Lytle BW: personal communication) (See Figure 1). These advances occurred at a time when the use of circulatory arrest was becoming more widely disseminated and the elephant trunk repair was being introduced (2,3). Not long after that, several pioneering aortic surgeons demonstrated in the mid-1990s that more extensive arch repairs were not only safe, but effective at promoting distal aortic remodeling in patients presenting with acute type 1 dissection (4-7). Nonetheless, over the last two and a half decades, the most common operation performed in this emergency setting is a supra-coronary ascending and hemi-arch interposition graft repair. The reluctance to perform more extensive repairs at the time of acute proximal aortic dissection is due to concerns that performing a bigger operation will increase the perioperative mortality (8-10).
Within the last decade, endovascular stent-graft devices have been widely adopted to treat thoracic aortic disease. Novel hybrid strategies combining the use of these devices with conventional open surgery show promise to simplify more extensive aortic repair at the time of acute dissection (11-19). With wider access to endovascular devices, brain protection strategies becoming more commonplace, and reproducible results with hybrid repairs, is only repairing the ascending aorta good enough in the modern era? I believe that the answer to this question is a resounding ‘no’. We should replace the arch and more in patients who present with acute DeBakey type 1 aortic dissection. I believe that embracing these newer techniques will allow us to make the incremental improvements in outcomes for acute proximal dissection that have not been seen since the mid-1980s.
New technology leads to new techniques
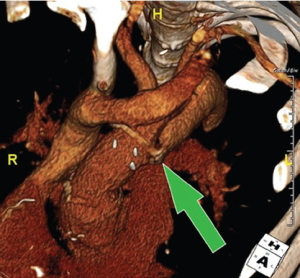
The use of stent-grafts in combination with open surgery has slowly been gaining favor for the treatment of patients with complex aortic pathology. Kato described the use of a graft with a distal stent as a modification of the conventional elephant trunk procedure, and Karck coined the term ‘frozen elephant trunk’ to describe the approach (5,11). Uchida developed another version of this technique using a stented graft sewn at the level distal to the left subclavian artery (12). Sun’s method of repair moved the suture line proximally with a fully stented device, and also included separate branch reconstruction of the arch vessels during antegrade brain perfusion (13). A specifically designed hybrid device (E-vita, Jotec, Germany) has been available in Europe for several years and is being evaluated as part of a multi-center study (14,15). Others have described the use of commercially available stent-grafts placed transfemorally or directly into the descending aorta at the time of proximal repair with reasonable success (16,17). The problem with the latter approach is that it may leave false lumen communications within the aortic arch at the level of the suture lines (18) (see Figure 2).
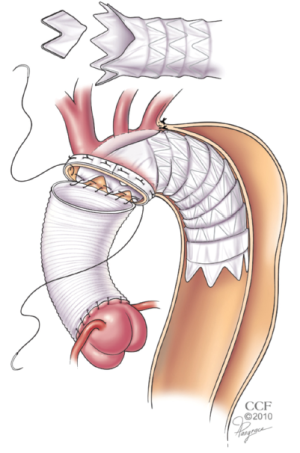
In the US, a technique including antegrade delivery of commercial stentgrafts and suturing has been described by others and us (19,20). I currently prefer a simplified approach to frozen elephant trunk repair at the time of acute DeBakey type I dissection which involves: antegrade stent-graft delivery, modification of the stent-graft within the aortic arch, and suture fixation within a single distal arch anastomosis, as this achieves a complete repair while limiting the circulatory arrest time (21) (Figure 3). Although all of these variations on a theme have been utilized safely, I believe that we need to develop a new standardized device and simplified technique to assure safety, consistent aortic remodeling, and more widespread adoption.
The acute risk argument against extended repair
Those in favor of maintaining the status quo argue that extending aortic repair will increase the operative risk of death. Patients who present with more extensive dissection involving multiple segments of the aorta (DeBakey type I) are at a higher risk of distal complications than those in whom the dissection is limited to the ascending aorta (DeBakey type II). This disparity occurs because the disease processes are different, and as such the operation should be tailored to the specific details of the pathology. It has long been recognized that it is important to not only consider the extent of dissection when planning a repair, but also to consider other details, such as the location of the entry tear.
In a review published in 1993, Drs James Kirklin and Nicholas Kouchoukos estimated that 20-30% of acute dissections require additional repair of the aortic arch. They recommended four indications for including the aortic arch in the repair: presence of the intimal tear in the arch, rupture of the arch, fragile tissue in the false lumen of the arch, and a fragmented false channel (22). In some surgical experiences the need to replace the arch during acute type A dissection repair increased the risk of surgery, but this has not been consistently demonstrated in the literature (4,23). The cause of death in many of these patients was related to ischemia or bleeding.
The acute risk argument in favor of extended repair
Although acute mortality has improved at several high volume centers, the risk of death is still predicted by the patient’s presentation. Patients presenting with malperfusion are at the highest risk, and this finding has been demonstrated in several series (24,25). In one study the presence of ischemia at the time of acute dissection increased mortality from 3% in those without ischemia, to over 25% in those with it (26).
This finding represents an opportunity for improvement. The addition of a stent-graft to the extended repair operation offers the potential to more rapidly reverse the malperfusion process. The cloth on the device covers any additional entry tears in the arch or proximal descending aorta and the radial force provided by the stent pushes open the true lumen. In our initial experience with this technique, 47% of patients presented with distal malperfusion and none of them suffered late loss of end-organ function (21). To maximize this opportunity, however, it is important to perform an angiogram at the end of the procedure to assess for any persistent distal malperfusion. Although the stentgraft addresses the dynamic obstructive pathophysiology, some patients will require additional procedures to address the static component of branch malperfusion (27). In our experience, four patients (30%) required additional branch vessel stenting for occlusion of the superior mesenteric artery, iliac artery (n=2), or left subclavian artery in the setting of prior internal mammary bypass grafting (21). By bringing the patients to a hybrid operating room for the frozen elephant trunk repair, the static malperfusion can be detected and treated without delay in the same setting, thereby avoiding the sequelae of ongoing ischemia after aortic repair.
Although the initial intent of performing an extended repair at the time of acute dissection has been to promote aortic remodeling and reduce the late complications associated with a patent false lumen, the potential to optimize true lumen perfusion and minimize the effects of distal malperfusion may prove to be the most important benefit of this approach. If we had a disease-specific device and a standardized approach, I expect we will see more widespread adoption as a surgical repair strategy.
Another benefit we have found with this approach is that the addition of the stent-graft in the arch and inclusion of the device within the distal anastomosis promotes hemostasis. The outward radial force against the fragile layers of tissue in the arch seem to promote false lumen thrombosis very early along that portion of the aorta and eliminate the bleeding that is sometimes seen with the conventional surgical approach, where the arch false lumen can be “boggy” from the persistent false lumen perfusion.
In the largest series to date including 148 acute type A dissection repairs, Sun and colleagues reported a mortality of only 4.7%, which compared favorably to a similar group of patients undergoing conventional repair who had a mortality of 6.1% (28). We have had no acute deaths to date in our series, which is now up to 26 patients. Others have reported similar safety with mortality rates in the single digits.
The late risk argument against extended repair
Some might argue that the initial operation on the acutely dissected aorta should be kept as simple as possible because there is always the option to return for a reoperation on the arch and/or distal aorta at a later elective setting. One argument against this line of thinking has already been addressed above: the more extended repair may be beneficial in the acute setting because of the benefits of optimized distal perfusion. The other is that there is very little data to support the assertion that a later reoperation on the distal aorta is safe. Estrera and colleagues described their experience with reoperations in patients who survived an acute type A dissection; a mortality rate of 11.1% was reported in 63 patients (29). This series only included 39 arches, with the rest being patients undergoing proximal aortic repair. No patients had descending or thoracoabdominal involvement. Many of these patients will require two operations: a total arch with elephant trunk stage 1 followed by distal repair of the descending or thoracoabdominal aorta, and therefore will be exposed to the combined risk of both procedures (30). In our series of patients requiring distal operation for chronic aortic dissection the perioperative mortality was 8% (31). We are currently studying our series of over 400 patients who required distal aortic operations after previous type A dissection and hope to shed some light on what that late risk entails. I anticipate our results will be reasonable but I am certain that most patients would prefer to avoid those second and third operations if possible.
As of yet, there is no data to confirm that the stentgraft-induced remodeling of the arch and proximal descending aorta will reduce the need for late reoperations, but it has been shown that a patent false lumen increases late risk of death and reoperation (32,33). It is also true that most patients who develop aneurysmal degeneration in the chronic phase will have this occur in the arch and the most proximal segment of the descending aorta (34). Also, a lot of late operations occur many years after surviving the acute event so we don’t fully understand the very long-term natural history of what occurs to the chronic residually dissected distal aorta. As we gain more experience at advanced centers with this extended approach, we should learn the true impact on survival and reoperation in these patients (35).
The late risk argument in favor of extended repair
The pioneering surgeon E. Stanley Crawford said of aortic dissection that “no patient should be considered cured of the disease”. It is estimated that about two-thirds of patients who survive an acute dissection have a persistent distal dissection (1,36). In the subset of patients presenting with DeBakey type I dissection the proportion may be higher. Of these, up to half will go on to require another operation or die from aortic associated causes. It has been shown that the patency of the false lumen is strongly associated with higher ongoing mortality and the need for reintervention (32,33). A review of the literature has demonstrated that the risk of late intervention ranges from 9-67% and the most common risk factors are young age, the presence or family history of a connective tissue disorder, a larger aortic diameter at the time of presentation, and patency of the false lumen (36).
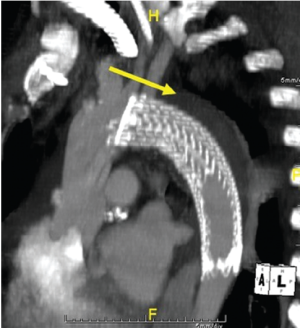
Extended hybrid repair combining a distal stent-graft with more conventional proximal repair has been shown to induce remodeling of the aortic arch and proximal descending aorta. Sun et al., had shown that the false lumen was thrombosed through the treated segment in 94% of 138 patients (28). Similarly, in the multicenter E-Vita trial, 89% of patients receiving the specially designed hybrid stent-graft device for acute dissection demonstrated false lumen thrombosis through the treated segment at late follow-up (15). Using our simplified frozen elephant trunk technique with an on-table modification to a commercially available stentgraft, we found that 88% of patients had thrombosis of the false lumen in the treated segment (21) (Figure 4).
Overcoming hurdles to implementing a new standard of care
Our center and several other prominent aortic centers have published reasonable outcomes with a hybrid extended approach to acute dissection. The techniques share the use of a stent-graft in combination with conventional open repair, but otherwise they have been quite varied. This approach demonstrates promise to make a difference in outcomes in both the acute and chronic phases of the disease, but will require much closer analysis of the results. Furthermore, there is a need for a disease-specific device and standardized simplified technique to assure consistent results and more widespread adoption.
Acknowledgements
Disclosure: The author declares no conflict of interest.
References
- Sabik JF, Lytle BW, Blackstone EH, et al. Long-term effectiveness of operations for ascending aortic dissections. J Thorac Cardiovasc Surg 2000;119:946-62.
- Borst HG. The birth of the elephant trunk technique. J Thorac Cardiovasc Surg 2013;145:44.
- Svensson LG. Rationale and technique for replacement of the ascending aorta, arch, and distal aorta using a modified elephant trunk procedure. J Card Surg 1992;7:301-12.
- Ando M, Nakajima N, Adachi S, et al. Simultaneous graft replacement of the ascending aorta and total aortic arch for type A aortic dissection. Ann Thorac Surg 1994;57:669-76.
- Kato M, Ohnishi K, Kaneko M, et al. New graft-implanting method for thoracic aortic aneurysm or dissection with a stented graft. Circulation 1996;94:II188-93.
- Pugliese P, Pessotto R, Santini F, et al. Risk of late reoperations in patients with acute type A aortic dissection: impact of a more radical surgical approach. Eur J Cardiothorac Surg 1998;13:576-80; discussion 580-1.
- Kazui T, Washiyama N, Muhammad BA, et al. Extended total arch replacement for acute type a aortic dissection: experience with seventy patients. J Thorac Cardiovasc Surg 2000;119:558-65.
- Sundt TM, Moon MR, DeOliviera N, et al. Contemporary results of total aortic arch replacement. J Card Surg 2004;19:235-9.
- Elefteriades JA. Editorial comment: extended resection in acute type A aortic dissection. Cardiol Clin 2010;28:349-50.
- Kouchoukos NT. The stented elephant trunk: is it an optimal strategy? J Card Surg 2009;24:702-3.
- Karck M, Chavan A, Hagl C, et al. The frozen elephant trunk technique: a new treatment for thoracic aortic aneurysms. J Thorac Cardiovasc Surg 2003;125:1550-3.
- Uchida N, Ishihara H, Shibamura H, et al. Midterm results of extensive primary repair of the thoracic aorta by means of total arch replacement with open stent graft placement for an acute type A aortic dissection. J Thorac Cardiovasc Surg 2006;131:862-7.
- Sun LZ, Qi RD, Chang Q, et al. Surgery for acute type A dissection using total arch replacement combined with stented elephant trunk implantation: experience with 107 patients. J Thorac Cardiovasc Surg 2009;138:1358-62.
- Jakob H, Tsagakis K, Tossios P, et al. Combining classic surgery with descending stent grafting for acute DeBakey type I dissection. Ann Thorac Surg 2008;86:95-101.
- Tsagakis K, Pacini D, Di Bartolomeo R, et al. Multicenter early experience with extended aortic repair in acute aortic dissection: is simultaneous descending stent grafting justified? J Thorac Cardiovasc Surg 2010;140:S116-20; discussion S142-S146.
- Fleck T, Hutschala D, Czerny M, et al. Combined surgical and endovascular treatment of acute aortic dissection type A: preliminary results. Ann Thorac Surg 2002;74:761-5; discussion 765-6.
- Mossop P, Nixon I, Oakes J, et al. Immediate “total” aortic true lumen expansion in type A and B acute aortic dissection after endovascular aortic endografting and GZSD bare stenting. J Thorac Cardiovasc Surg 2007;134:1360-2.
- Panos A, Kalangos A, Christofilopoulos P, et al. Combined surgical and endovascular treatment of aortic type A dissection. Ann Thorac Surg 2005;80:1087-90.
- Pochettino A, Brinkman WT, Moeller P, et al. Antegrade thoracic stent grafting during repair of acute DeBakey I dissection prevents development of thoracoabdominal aortic aneurysms. Ann Thorac Surg 2009;88:482-9; discussion 489-90.
- Roselli EE, Soltesz EG, Mastracci T, et al. Antegrade delivery of stent grafts to treat complex thoracic aortic disease. Ann Thorac Surg 2010;90:539-46.
- Roselli EE, Rafael A, Soltesz EG, et al. Simplified frozen elephant trunk repair for acute DeBakey type I dissection. J Thorac Cardiovasc Surg 2013;145:S197-201.
- Kirklin JW, Kouchoukos NT. When and how to include arch repair in patients with acute dissections involving the ascending aorta. Semin Thorac Cardiovasc Surg 1993;5:27-32.
- Ochiai Y, Imoto Y, Sakamoto M, et al. Long-term effectiveness of total arch replacement for type A aortic dissection. Ann Thorac Surg 2005;80:1297-302.
- Geirsson A, Szeto WY, Pochettino A, et al. Significance of malperfusion syndromes prior to contemporary surgical repair for acute type A dissection: outcomes and need for additional revascularizations. Eur J Cardiothorac Surg 2007;32:255-62.
- Santini F, Montalbano G, Casali G, et al. Clinical presentation is the main predictor of in-hospital death for patients with acute type A aortic dissection admitted for surgical treatment: a 25 years experience. Int J Cardiol 2007;115:305-11.
- Augoustides JG, Geirsson A, Szeto WY, et al. Observational study of mortality risk stratification by ischemic presentation in patients with acute type A aortic dissection: the Penn classification. Nat Clin Pract Cardiovasc Med 2009;6:140-6.
- Patel HJ, Williams DM, Dasika NL, et al. Operative delay for peripheral malperfusion syndrome in acute type A aortic dissection: a long-term analysis. J Thorac Cardiovasc Surg 2008;135:1288-95; discussion 1295-6.
- Sun L, Qi R, Zhu J, et al. Total arch replacement combined with stented elephant trunk implantation: a new “standard” therapy for type a dissection involving repair of the aortic arch? Circulation 2011;123:971-8.
- Estrera AL, Miller CC 3rd, Villa MA, et al. Proximal reoperations after repaired acute type A aortic dissection. Ann Thorac Surg 2007;83:1603-8; discussion 1608-9.
- Greenberg R, Roselli E. Commentary on “Surgical repair of extensive aortic aneurysms”. Perspect Vasc Surg Endovasc Ther 2005;17:223-6.
- Pujara AC, Roselli EE, Hernandez AV, et al. Open repair of chronic distal aortic dissection in the endovascular era: Implications for disease management. J Thorac Cardiovasc Surg 2012;144:866-73.
- Fattouch K, Sampognaro R, Navarra E, et al. Long-term results after repair of type a acute aortic dissection according to false lumen patency. Ann Thorac Surg 2009;88:1244-50.
- Halstead JC, Meier M, Etz C, et al. The fate of the distal aorta after repair of acute type A aortic dissection. J Thorac Cardiovasc Surg 2007;133:127-35.
- Tsai TT, Evangelista A, Nienaber CA, et al. Long-term survival in patients presenting with type A acute aortic dissection: insights from the International Registry of Acute Aortic Dissection (IRAD). Circulation 2006;114:I350-6.
- Murzi M, Tiwari KK, Farneti PA, et al. Might type A acute dissection repair with the addition of a frozen elephant trunk improve long-term survival compared to standard repair? Interact Cardiovasc Thorac Surg 2010;11:98-102.
- Subramanian S, Roselli EE. Thoracic aortic dissection: long-term results of endovascular and open repair. Semin Vasc Surg 2009;22:61-8.





