A reappraisal of retrograde cerebral perfusion
Introduction
Aortic arch surgery has advanced remarkably over the past few decades. Brain protection during the interval in which normal circulation is interrupted is the most critical. As well as preventing ischemic brain injury, the prevention of stroke due to embolization of thrombus or atheromatous debris is essential. Furthermore, many factors are associated with poor neurological outcomes, for example, emergency status, aortic dissection with malperfusion, degree of systemic atheromatous arterial disease, history of cerebrovascular accidents, and renal insufficiency. Therefore, aortic arch surgery is still challenging for surgeons and peri-operative neurological injury remains significant.
In terms of brain and organ protection, antegrade cerebral perfusion (ACP) and hypothermic circulatory arrest (HCA) are the two conventional methods which were utilized for aortic arch surgery in the past. HCA has been widely used since acceptable surgical results were published by Griepp et al. in 1975 (1-9). The use of HCA simplified the operative procedure and removed the need for extensive manipulation of the brachiocephalic branches. However, the operation afforded by HCA is imperfect and of limited duration (1-5). The clinical application of continuous retrograde cerebral perfusion (RCP) with HCA for aortic arch surgery was first shown by Ueda and colleagues in 1990 (10). Thereafter, many clinical papers demonstrated the efficacy of RCP and identified a reduction in early mortality and morbidity (11-23). RCP entered routine use as an adjunct for prolonged HCA in the 1990s. Many of those RCP studies reported favorable results compared to historical control series. Although some evidence for enhanced protection by RCP is compelling, it is unclear whether the perceived improvements in mortality and morbidity reflect the direct benefit of RCP or is the result of overall improved surgical and anesthetic techniques (24,25).
History of retrograde perfusion
In 1980, Mills and Ochsner (26) originally used RCP to treat accidental air embolisms during cardiopulmonary bypass. In 1982, Lemole and colleagues (27) reported the treatment of an acute type A dissection using an intraluminal sutureless graft. They described intermittent RCP only briefly in a part of the discussion section, with a schema of the perfusion circuit (Figure 1). RCP into the superior vena cava (SVC) was used every 20 minutes during HCA.
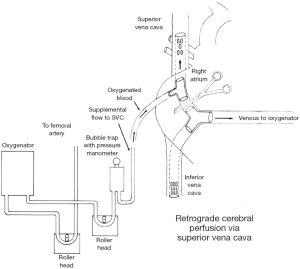
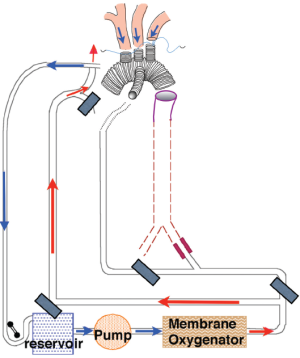
In 1986, unaware of Lemole’s paper, RCP was introduced by Ueda and colleagues independently (10). RCP was later extended from an intermittent administration to continuous administration (Figure 2) (10-12). RCP flow was always regulated to maintain a pressure of less than 20 mmHg in the internal jugular vein. This was found to provide satisfactory RCP flow in experimental animal models by Usui et al. (28). Flow-regulated RCP is not recommended; instead pressure-regulated RCP is favored to reduce the risk of brain edema with sustained jugular vein pressure over 20 mmHg.
Anatomy of the jugular veins in man and mammals
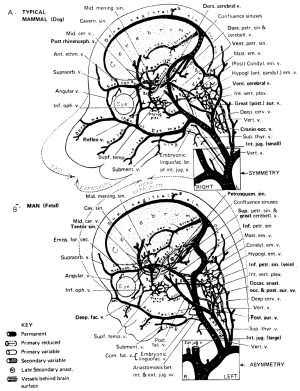
The venous drainage from the brain in humans differ significantly from that in non-primate mammals. Kalbag (29) precisely described the anatomy and embryology of the cerebral venous system in “Handbook of Neurology Vol. 11” in 1972. Figures entitled “The development of the cranial venous system in man, from the viewpoint of comparative anatomy” (Figure 3) by Padget (30) were cited in this chapter. The head and neck are primarily drained by the internal jugular vein which remains predominant in man while in most other mammals, owing to the greater development of the face and neck relative to the brain, it is the external jugular vein that predominates. As the external jugular veins have many valves, venous regurgitation to the head is restricted, thereby allowing these non-primate mammals to freely eat and drink while lowering their heads.
On the other hand, the internal jugular veins, which have jugular bulbs with a remnant of venous valves, are the main drainage from the brain in humans. The human internal jugular veins are valved in approximately 90% individuals and studies in cadavers suggest that the valves are competent in 85% (31).
Investigations of RCP pressure and flow
The relationship between perfusion pressure, flow, and metabolism during RCP were investigated. Several papers have consistently demonstrated that RCP via SVC in a variety of animal models delivers only a small proportion of ACP flow in an uneven distribution (32-35). In 1995, Boeckstaens et al. measured the cerebral blood flow generated by RCP using a colored microsphere technique in primates (32). After 60 minutes of RCP delivered into the internal jugular veins at a pressure of 20 mmHg, cerebral blood flow was only 1% of antegrade values. However, as the inferior vena cava (IVC) was allowed to drain freely during the perfusion, preferential perfusion of low resistance venous collateral channels may have accounted for the low flows encountered. The relevance of such studies to human anatomy depends on accurate demonstration of RCP flow.
de Brux et al. (31) investigated blood distribution of RCP by colored latex injection into the SVC in adult cadavers. Despite valves in the internal jugular vein, latex injections into cadaver internal jugular veins following instrumentation to render the valves incompetent may still reach the venous sinuses and cerebral venous. Collateral venous systems with lower resistance than the valved internal jugular vein, particularly via the azygos vein, may be responsible for the majority of blood perfusing the brain retrogradely. The status of IVC drainage may have an important role in RCP delivery. Prevention of low pressure run-off via azygos-IVC collaterals may increase the amount of blood flow reaching the brain.
In canine studies with RCP delivery via the maxillary veins (therefore bypassing all the external jugular vein valves), Usui et al. (28) demonstrated that return of blood into the aorta was maximal when jugular pressure was 25 mmHg, with a linear relationship between flow and pressure in the range of 15 and 25 mmHg. Rising venous pressure above this did not augment flow despite a rise in cerebrospinal fluid pressure. Nojima et al. (36) also found that retrograde flow increased with venous pressures up to 30 mmHg, and significant cerebral edema occurred at 30 mmHg. High SVC pressure has been shown to be damaging, most likely by causing cerebral edema and raised intracranial pressure. They demonstrated that aortic effluent blood volume from brachiocephalic branches was increased if IVC drainage was occluded.
Kawata and colleagues (37,38) introduced a novel RCP method with intermittent pressure augmentation for cerebral protection during aortic surgery. In their animal study, the effect of such brain protection was reinforced by raising the RCP pressure intermittently (every 30 seconds) from 15 mmHg to 45 mmHg in less than 120 minutes HCA at 18 °C. Intermittent augmented pressure effectively dilates and “opens” the cerebral vessels, thereby enabling adequate blood supply to reach the brain while also minimizing brain damage.
Recently, Yang and colleagues (39) investigated the efficacy of a modified RCP protocol by magnetic resonance spectroscopy to track the changes of brain high-energy phosphates. A modified protocol of RCP in pigs with non-occlusion of the IVC, higher perfusion pressure at 40-50 mmHg, and pH-stat strategy could improve brain tissue perfusion and oxygenation.
Chronic porcine model of RCP
Safi et al. used a porcine model of RCP (40). They established three groups of 5 pigs each: group A (control) underwent cardiopulmonary bypass and normothermic circulatory arrest for 1 hour, group B underwent cardiopulmonary bypass and profound HCA (15 °C nasopharyngeal) for 1 hour, and group C underwent the same procedure as group B plus RCP. None of the animals awoke in group A (normothemia). In group B (HCA only), 2 of 5 did not wake, 3 of 5 woke but were unable to stand. All of the group C (HCA with RCP) pigs awoke, 4 of 5 were able to stand, and 1 that was unable to stand could move all limbs. The neurological evaluation of group B showed significantly lower Tarlov scores than group C (P=0.009). Group B had a mean wake-up time of 124.6±4.6 versus 29.2±5.1 minutes in group C (P=0.009). The late phase circulatory arrest brain oxygenation decreased by 46.0±13.9% in group B, but increased by 26.1±5.4% in group C (P=0.0013). The rewarming jugular venous O2 saturation in group B was 30.8±2.5% versus 56.0±4.4% in group C (P=0.0011). They concluded that RCP combined with profound HCA significantly reduces neurologic dysfunction, providing superior brain protection than HCA alone in pigs.
Yerlioglu et al. used RCP in a porcine model to evaluate the efficacy of RCP in mitigating the effects of particulate cerebral embolism occurring during cardiac surgery (41). Following embolisation at 20 °C with 250-750 µm albumin-coated polystyrene microspheres, cerebral perfusion was maintained antegradely or retrogradely via the SVC. They speculated that RCP, in addition to its usefulness as an adjunct to HCA, is attractive as a potential means of preventing air and particulate emboli which are the major causes of permanent neurologic injury after cardiac and aortic surgery in adults. They concluded that some pigs recover after embolization and RCP with either minimal or no cerebral injury. The complete recovery of these pigs, without any histopathological evidence of cerebral injury in contrast to the almost invariable neurological impairment and histopathological abnormalities in the antegrade embolism group, suggests that RCP provides some degree of cerebral protection after embolization in the ascending aorta. However, pigs perfused with RCP pressures greater than 40 mmHg had worse neurological outcomes. As a result, gradually instituting RCP and maintaining the SVC pressures at a level less than 40 mmHg seem to improve the results.
Juvonen and colleagues (42) developed a chronic porcine model to evaluate the efficacy of RCP. Sixty-two pigs were randomly assigned to undergo one of the following for 90 minutes at 20 °C: ACP, conventional RCP, RCP with occlusion of the inferior vena cava (RCP-O), or HCA with the head packed in ice. Complete behavioral recovery was seen in all surviving animals by day 5 after ACP or RCP, but in only 83% after RCP-O and 50% after HCA (P=0.001). They demonstrated that conventional RCP without inferior vena caval occlusion results in a significantly better outcome than RCP-O after prolonged HCA, despite more efficient cerebral perfusion during RCP-O, and also provides cerebral protection superior to prolonged HCA alone.
Comments
Various experimental studies on animals have been performed to evaluate the efficacy and limitations of RCP. Published studies revealed that RCP does not provide a sufficient amount of blood flow or oxygen substrate to the brain. However, the interpretation of these findings must bear in mind the different developmental anatomy of the jugular venous system in man compared with other mammals.
Furthermore, there were several controversial factors in the experimental protocols, such as an extended HCA with RCP time up to 90 to 120 minutes and body temperature of 20 °C instead of 18 °C. These experimental protocols seemed to be designed to lead the conclusion of the inferiority of RCP in comparison to ACP. However, it should be appreciated that RCP combined with HCA, even in those intense protocols, revealed a better neuroprotective effect than HCA alone (40-43).
Intraoperative investigation of RCP flow
Human studies of RCP are less clear; there are anecdotal reports of cerebral edema when perfusion pressures exceeding 25 mmHg are adopted. Collapsed cortical veins or functional jugular venous valves may restrict flow at the frequently recommended maximum pressure of 25 mmHg. Ganzel et al. (44) have shown that with extensive intraoperative monitoring RCP flow can be safely titrated, with higher driving pressures dependent upon demonstration of a reversal of Doppler flow signal in the middle cerebral artery. Using multi-modality neurophysiological monitoring they found no evidence of cerebral edema during RCP if SVC pressure was kept at 25 mmHg. Estrera et al. (45) described the detection of RCP flow in the middle cerebral arteries during aortic arch surgery. The RCP flow rate was varied depending on the information obtained from bilateral power mode transcranial Doppler ultrasound and bilateral near-infrared spectroscopy (cerebral oximetry). The adequacy of RCP flow was determined by the presence of reversed blood flow in the middle cerebral arteries when power mode transcranial Doppler ultrasound was used. Although a higher “opening” pressure is required, the maintenance flow rate is often below 500 mL/min, maintaining the pressure in the SVC line below 25 mmHg. The information obtained from power mode transcranial Doppler ultrasound was correlated with information obtained with from near-infrared spectroscopy.
Paganao et al. (46) injected the cerebral perfusion study agent 99mTechnetium labelled D,L-hexamethyl propylene amine oxime (99mTc-HMPAO) into the bypass reservoir at commencement of RCP. Continuous intra-operative gamma camera cerebral imaging revealed gradual accumulation of 99mTc-HMPAO firstly within the jugular bulb, followed by the sagittal and transverse sinuses and subsequently homogenous distribution throughout the gray and white matter. However, quantification of cerebral blood flow by this method was not possible but cumulative activity counts suggested that the actual blood flow was small and only a fraction of that obtained by antegrade perfusion.
Clinical outcomes of aortic arch surgery using RCP
Ueda and colleagues (23) published a retrospective analysis of 249 patients who underwent aortic arch surgery at three Japanese hospitals, where HCA and RCP were used as a routine adjunct, between 1994 and 1996. The pathology of the aortic arch was atherosclerotic aneurysm in 133 patients and dissection in 116. Seventy patients had surgery on an emergency basis. The hospital mortality was 25/249 (10%). Stroke developed in 11 patients (4%). The median duration of RCP was 46 minutes (range, 5 to 95 minutes). Multivariate logistic analysis revealed that pump time (P=0.0001), age (P=0.0001), and RCP time (P=0.052) were the most significant risk factors. The risk factors for mortality and neurological morbidity were pump time (P=0.0001), age (P=0.0002), urgency of surgery (P=0.07), and RCP time (P=0.15).
Coselli et al. (20) published their results of aortic arch surgery from 1987 through 1997 using HCA with RCP in 305 patients and without RCP in 204 patients. The in-hospital morality of 3.9% (12 patients) was significantly improved with RCP. In those without RCP, the in-hospital mortality was 17.2% (35 patients; P=0.001). The incidence of permanent stroke in patients undergoing RCP was 2.6% (8 patients), and the incidence for those without RCP was 6.4% (13 patients; P=0.037). The variables that were associated with early mortality in the patients with RCP were atherosclerotic heart disease, concomitant coronary artery bypass, aortic cross clamp time, pump time and sepsis. In this retrospective analysis of a large clinical series, RCP was found to significantly and favorably influence in-hospital mortality and the incidence of permanent stroke, although the period of HCA may be tolerable in most patients.
In 1997, Safi and associates (19) demonstrated that the overall 30-day mortality rate was 6% and the incidence of stroke was 4% in 161 patients who underwent surgery for aneurysms of the ascending aorta and transverse arch using HCA and RCP. The use of RCP had a protective effect against stroke (3 of 120 patients or 3%) in comparison to the absence of RCP (4 of 41 patients or 9%; P<0.049), and this phenomenon was most significant in patients older than 70 years. The cardiopulmonary bypass time was the sole factor found to be associated with an increased risk of stroke and mortality.
Thereafter, Safi et al. (47) revised their surgical results in 2011. They collected a large retrospective dataset from 1991 to 2010, and conducted comprehensive analysis on 1,193 patients who underwent surgery for the ascending aorta and arch. The 30-day mortality rate was 9.3% and the overall stroke rate was 3%. In univariate analysis of risk factors for stroke, the stroke rate was 2.8% with and 4.2% without retrograde cerebral perfusion (P=0.30), but when circulatory arrest time exceeded 40 minutes, the stroke rate was 1.7% with and 30% without retrograde cerebral perfusion (P=0.002). RCP demonstrated a protective effect against mortality and stroke. They concluded that RCP was associated with a trend towards reduced incidence of hospital mortality and, in patients receiving prolonged hypothermic circulatory arrest, reduced incidence of stroke.
Okita and associates (22) reported similar results and concluded that prolonged HCA and RCP for longer than 60 minutes was not a risk factor for mortality or stroke in patients who underwent aortic arch surgery. However, the prevalence of transient delirium necessitates further investigation. Their logistic regression analysis demonstrated that the risk factors for mortality were ruptured aneurysm, chronic obstructive pulmonary disease, arterial cannulation in the ascending aorta, and stroke.
Okita et al. (48) also conducted a prospective comparative study of brain protection during HCA with RCP or ACP. Sixty consecutive patients who underwent total arch replacement were allocated alternately into two groups: RCP and ACP, each with 30 patients. Hospital mortality occurred in 2 patients in each group. New strokes occurred in 1 (3.3%) of the RCP group and in 2 (6.6%) of the ACP group (P=0.6). Both methods of brain protection for patients undergoing total arch replacement resulted in acceptable levels of mortality and morbidity. The incidence of transient neurological dysfunction was significantly higher in the RCP group than in the ACP group (10 patients, 33.3% vs. 4 patients, 13.3%; P=0.05). Except in patients with strokes, S-100b values were not different in the two groups. There were no intergroup differences in the scores of memory decline, orientation or intellectual function.
Svensson and colleagues (49) conducted a prospective randomized neurocognitive and S-100 study of HCA, RCP, and ACP for aortic arch surgery. Thirty patients who underwent aortic arch operations were randomly assigned to three equal groups for HCA, ACP, and RCP. All patients underwent a battery of 14 neurocognitive tests resulting in 51 subscores per patient at each of four test intervals. Serum S-100 protein levels were measured at more than 12 time intervals, which included before cannulation for cardiopulmonary bypass, going onto cardiopulmonary bypass, at the end of cooling before circulatory arrest, immediately after circulatory arrest, at the end of rewarming, at the conclusion of cardiopulmonary bypass, leaving the operating room, and then at 6, 12, 18, 24, and 48 hours postoperatively. For the randomized patients, the survival rate was 100% and no patient suffered a stroke or seizure. Circulatory arrest (HCA) times were not different (HCA vs. ACP vs. RCP) for 11 total arch repairs (including 6 elephant trunk; mean, 41.4±15 minutes). The total circulatory arrest time (P=0.01) and cardiopulmonary bypass time (P=0.057) correlated with the peak serum S-100 levels. Circulatory arrest time correlated inversely with the following neurocognitive scores (P=0.05). They recommend that surgeons continue to use HCA by the well established techniques, and RCP or ACP be added on a selective basis according to the expected operative procedure, namely RCP when a lot of potential embolic material is expected and ACP when prolonged HCA times may occur. Although this study has failed to show added neurocognitive protective benefits with these techniques, in a larger series of patients and with a greater number of strokes there could potentially be some benefit in stroke reduction.
A number of papers demonstrated a spectrum of beneficial, neutral, and detrimental effects of RCP in humans and in experimental animal models. In 2001, Reich and colleagues published a systematic review of evidence-based literature concerning RCP (50). They summarized that the early clinical and laboratory results regarding RCP for thoracic aortic surgery were promising, however it remains unclear whether RCP provides effective cerebral perfusion, metabolic support, washout of embolic material, and improved neurological and neuropsychological outcomes.
In 2004, Barnard et al. (51) published a systematic review on brain protection during HCA to determine whether patients undergoing aortic arch surgery benefit from ACP or RCP to reduce neurological injury or mortality. Altogether 408 papers were found using the reported search, of which 16 papers presented the best evidence to answer the clinical question. The author, journal, date and country of publication, patient group studied, study type, relevant outcomes, results, and study weaknesses of these papers were tabulated. They concluded that ACP is superior as an adjunct to HCA when compared to RCP or HCA alone, although clinical evidence for this from prospective clinical trials is weak.
In 2012, Usui and colleagues (52) conducted a comparative study to evaluate up-to-date clinical outcomes of aortic arch surgery between ACP and RCP based on the Japan Adult Cardiovascular Surgery Database. The subjects were confined to cases undergone electively with ACP or RCP for non-dissection aneurysms in the ascending aorta and aortic arch between 2005 and 2008 from 13,467 aortic surgeries. There were 2,209 ACP cases and 583 RCP cases. A risk-adjusted comparison based on 30-day mortality, operative mortality, and major morbidity was assessed by a multivariable logistic regression analysis. A conditional logistic regression analysis was also conducted in 499 propensity matched-pairs with ACP and RCP. A risk-adjusted analysis showed no significant differences between the ACP and RCP groups regarding 30-day mortality (3.5% vs. 2.6%), operative mortality (5.3% vs. 4.1%), or stroke (6.8% vs. 3.1%). Propensity-matched pairs also revealed no significant differences between ACP and RCP regarding 30-day mortality (3.4% vs. 2.4%), operative mortality (3.8% vs. 3.4%), or stroke rate (5.0% vs. 3.0%); however, RCP resulted in a significantly higher rate of transient neurological dysfunction (3.0% vs. 5.8%) and need for dialysis (1.6% vs. 4.2%).
Estrera and Safi have consistently reported the beneficial impact of RCP for aortic arch surgery with the largest number of patients (53-57). They recommended the use of transcranial Doppler scanning to direct the retrograde perfusion, as well as cerebral oximetry by bilateral near-infrared spectroscopy to demonstrate RCP in the cerebral vessels. They preferred to use RCP with HCA, although RCP was not used in all case. They used ACP, combination of ACP and RCP, or HCA alone, depending on surgical strategy and pathology. “Integrated cerebral perfusion” for HCA during transverse aortic arch repairs has been advocated since 2010 (55).
Conclusions
The significant limitations of HCA to protect the brain during the aortic arch replacement has led to the introduction of RCP as an adjunct to extended the ‘safe’ duration of arrest period and to eliminate embolisation of air and debris. The technical simplicity of RCP together with a highly favorable impact upon both stroke rate and survival after aortic arch surgery justifies the continued clinical use of RCP in patients requiring HCA lasting about 40 to 60 minutes.
RCP is a beneficial adjunct for aortic arch surgery with 40 to 60 minutes HCA. However, a conversion from ACP to RCP as the adjunct of choice for brain protection is not advocated. ACP is preferable for the complex and extensive aortic arch surgery expected with prolonged HCA. We should realize that the technique of how we perform these adjuncts is important and that RCP still remains a valuable adjunct for cerebral protection in patients with acute dissection or atheromatous cervical branches.
Acknowledgements
Disclosure: The author declares no conflict of interest.
References
- Griepp RB, Stinson EB, Hollingsworth JF, et al. Prosthetic replacement of the aortic arch. J Thorac Cardiovasc Surg 1975;70:1051-63. [PubMed]
- Crawford ES, Snyder DM. Treatment of aneurysms of the aortic arch. A progress report. J Thorac Cardiovasc Surg 1983;85:237-46. [PubMed]
- Coselli JS, Crawford ES, Beall AC Jr, et al. Determination of brain temperatures for safe circulatory arrest during cardiovascular operation. Ann Thorac Surg 1988;45:638-42. [PubMed]
- Crawford ES, Svensson LG, Coselli JS, et al. Surgical treatment of aneurysm and/or dissection of the ascending aorta, transverse aortic arch, and ascending aorta and transverse aortic arch. Factors influencing survival in 717 patients. J Thorac Cardiovasc Surg 1989;98:659-73; discussion 673-4. [PubMed]
- Svensson LG, Crawford ES, Hess KR, et al. Deep hypothermia with circulatory arrest. Determinants of stroke and early mortality in 656 patients. J Thorac Cardiovasc Surg 1993;106:19-28; discussion 28-31. [PubMed]
- Kouchoukos NT, Abbound N, Klausing WR. Perfusion for thoracic aortic surgery. In: Gravlee GP, Davis RE, Utley JR. eds. Cardiopulmonary bypass. Principles and practice. Baltimore: Williams & Wilkins, 1993: 636-54.
- Borst HG, Laas J. Surgical treatment of thoracic aortic aneurysms. In: Advance in Cardiac Surgery. St. Louis: Mosby-Year Book 1993:47-87.
- Ergin MA, Griepp EB, Lansman SL, et al. Hypothermic circulatory arrest and other methods of cerebral protection during operations on the thoracic aorta. J Card Surg 1994;9:525-37. [PubMed]
- Kouchoukos NT, Dougenis D. Surgery of the thoracic aorta. N Engl J Med 1997;336:1876-88. [PubMed]
- Ueda Y, Miki S, Kusuhara K, et al. Surgical treatment of aneurysm or dissection involving the ascending aorta and aortic arch, utilizing circulatory arrest and retrograde cerebral perfusion. J Cardiovasc Surg (Torino) 1990;31:553-8. [PubMed]
- Ueda Y, Miki S, Kusuhara K, et al. Deep hypothermic systemic circulatory arrest and continuous retrograde cerebral perfusion for surgery of aortic arch aneurysm. Eur J Cardiothorac Surg 1992;6:36-41; discussion 42. [PubMed]
- Ueda Y, Miki S, Okita Y, et al. Protective effect of continuous retrograde cerebral perfusion on the brain during deep hypothermic systemic circulatory arrest. J Card Surg 1994;9:584-94; discussion 594-5. [PubMed]
- Takamoto S, Matsuda T, Harada M, et al. Simple hypothermic retrograde cerebral perfusion during aortic arch replacement. A preliminary report on two successful cases. J Thorac Cardiovasc Surg 1992;104:1106-9. [PubMed]
- Coselli JS. Retrograde cerebral perfusion via a superior vena caval cannula for aortic arch aneurysm operations. Ann Thorac Surg 1994;57:1668-9. [PubMed]
- Bavaria JE, Woo YJ, Hall RA, et al. Retrograde cerebral and distal aortic perfusion during ascending and thoracoabdominal aortic operations. Ann Thorac Surg 1995;60:345-52; discussion 352-3. [PubMed]
- Deeb GM, Jenkins E, Bolling SF, et al. Retrograde cerebral perfusion during hypothermic circulatory arrest reduces neurologic morbidity. J Thorac Cardiovasc Surg 1995;109:259-68. [PubMed]
- Lytle BW, McCarthy PM, Meaney KM, et al. Systemic hypothermia and circulatory arrest combined with arterial perfusion of the superior vena cava. Effective intraoperative cerebral protection. J Thorac Cardiovasc Surg 1995;109:738-43. [PubMed]
- Usui A, Abe T, Murase M. Early clinical results of retrograde cerebral perfusion for aortic arch operations in Japan. Ann Thorac Surg 1996;62:94-103; discussion 103-4. [PubMed]
- Safi HJ, Letsou GV, Iliopoulos DC, et al. Impact of retrograde cerebral perfusion on ascending aortic and arch aneurysm repair. Ann Thorac Surg 1997;63:1601-7. [PubMed]
- Coselli JS. Retrograde cerebral perfusion in surgery for aortic arch aneurysms. In: Ennker J, Coselli JS, Hetzer R. eds. Cerebral protection in cerebraovascular and aortic surgery. Darmstadt: Steinkopf Verlag, 1997:239-49.
- Bavaria JE, Pochettino A. Retrograde cerebral perfusion (RCP) in aortic arch surgery: efficacy and possible mechanisms of brain protection. Semin Thorac Cardiovasc Surg 1997;9:222-32. [PubMed]
- Okita Y, Takamoto S, Ando M, et al. Mortality and cerebral outcome in patients who underwent aortic arch operations using deep hypothermic circulatory arrest with retrograde cerebral perfusion: no relation of early death, stroke, and delirium to the duration of circulatory arrest. J Thorac Cardiovasc Surg 1998;115:129-38. [PubMed]
- Ueda Y, Okita Y, Aomi S, et al. Retrograde cerebral perfusion for aortic arch surgery: analysis of risk factors. Ann Thorac Surg 1999;67:1879-82; discussion 1891-4.
- Bonser RS, Wong CH. Retrograde Perfusion. In: Newman SP, Harrison MJ. eds. Brain and Cardiac Surgery: Causes of Neurological Complications and their Prevention. Reading, Harwood Academic Publishers, 2000:199-208.
- Ueda Y. What is the best method for brain protection in surgery of the aortic arch? Retrograde cerebral perfusion. Cardiol Clin 2010;28:371-9. [PubMed]
- Mills NL, Ochsner JL. Massive air embolism during cardiopulmonary bypass. Causes, prevention, and management. J Thorac Cardiovasc Surg 1980;80:708-17. [PubMed]
- Lemole GM, Strong MD, Spagna PM, et al. Improved results for dissecting aneurysms. Intraluminal sutureless prosthesis. J Thorac Cardiovasc Surg 1982;83:249-55. [PubMed]
- Usui A, Oohara K, Liu TL, et al. Determination of optimum retrograde cerebral perfusion conditions. J Thorac Cardiovasc Surg 1994;107:300-8. [PubMed]
- Kalbag RM. Anatomy and embryology of the cerebral venous system. In: Vinken PJ, Bruyn GW. eds. Handbook of Neurology Vol. 11, Vascular disease of the nervous system. Part I. Amsterdam: North-Holland Publishing Company,1972:58.
- Padget DH. The development of the cranial venous system in man, from the viewpoint of comparative anatomy. Contr Embryo Carneg Instn 1957;36:81-140.
- de Brux JL, Subayi JB, Pegis JD, et al. Retrograde cerebral perfusion: anatomic study of the distribution of blood to the brain. Ann Thorac Surg 1995;60:1294-8. [PubMed]
- Boeckxstaens CJ, Flameng WJ. Retrograde cerebral perfusion does not perfuse the brain in nonhuman primates. Ann Thorac Surg 1995;60:319-27; discussion 327-8. [PubMed]
- Tanoue Y, Tominaga R, Ochiai Y, et al. Comparative study of retrograde and selective cerebral perfusion with transcranial Doppler. Ann Thorac Surg 1999;67:672-5. [PubMed]
- Ehrlich MP, Hagl C, McCullough JN, et al. Retrograde cerebral perfusion provides negligible flow through brain capillaries in the pig. J Thorac Cardiovasc Surg 2001;122:331-8. [PubMed]
- Bonser RS, Wong CH, Harrington D, et al. Failure of retrograde cerebral perfusion to attenuate metabolic changes associated with hypothermic circulatory arrest. J Thorac Cardiovasc Surg 2002;123:943-50. [PubMed]
- Nojima T, Mori A, Watarida S, et al. Cerebral metabolism and effects of pulsatile flow during retrograde cerebral perfusion. J Cardiovasc Surg (Torino) 1993;34:483-92. [PubMed]
- Kawata M, Takamoto S, Kitahori K, et al. Intermittent pressure augmentation during retrograde cerebral perfusion under moderate hypothermia provides adequate neuroprotection: an experimental study. J Thorac Cardiovasc Surg 2006;132:80-8. [PubMed]
- Kawata M, Sekino M, Takamoto S, et al. Retrograde cerebral perfusion with intermittent pressure augmentation provides adequate neuroprotection: diffusion- and perfusion-weighted magnetic resonance imaging study in an experimental canine model. J Thorac Cardiovasc Surg 2006;132:933-40. [PubMed]
- Yang Y, Yang L, Sun J, et al. A modified protocol for retrograde cerebral perfusion: magnetic resonance spectroscopy in pigs. Eur J Cardiothorac Surg 2013;43:1065-71. [PubMed]
- Safi HJ, Iliopoulos DC, Gopinath SP, et al. Retrograde cerebral perfusion during profound hypothermia and circulatory arrest in pigs. Ann Thorac Surg 1995;59:1107-12. [PubMed]
- Yerlioglu ME, Wolfe D, Mezrow CK, et al. The effect of retrograde cerebral perfusion after particulate embolization to the brain. J Thorac Cardiovasc Surg 1995;110:1470-84; discussion 1484-5. [PubMed]
- Juvonen T, Zhang N, Wolfe D, et al. Retrograde cerebral perfusion enhances cerebral protection during prolonged hypothermic circulatory arrest: a study in a chronic porcine model. Ann Thorac Surg 1998;66:38-50. [PubMed]
- Anttila V, Kiviluoma K, Pokela M, et al. Cold retrograde cerebral perfusion improves cerebral protection during moderate hypothermic circulatory arrest: A long-term study in a porcine model. J Thorac Cardiovasc Surg 1999;118:938-45. [PubMed]
- Ganzel BL, Edmonds HL Jr, Pank JR, et al. Neurophysiologic monitoring to assure delivery of retrograde cerebral perfusion. J Thorac Cardiovasc Surg 1997;113:748-55; discussion 755-7. [PubMed]
- Estrera AL, Miller CC 3rd, Lee TY, et al. Ascending and transverse aortic arch repair: the impact of retrograde cerebral perfusion. Circulation 2008;118:S160-6. [PubMed]
- Pagano D, Boivin CM, Faroqui MH, et al. Retrograde perfusion through the superior vena cava perfuses the brain in human beings. J Thorac Cardiovasc Surg 1996;111:270-2. [PubMed]
- Safi HJ, Miller CC 3rd, Lee TY, et al. Repair of ascending and transverse aortic arch. J Thorac Cardiovasc Surg 2011;142:630-3. [PubMed]
- Okita Y, Minatoya K, Tagusari O, et al. Prospective comparative study of brain protection in total aortic arch replacement: deep hypothermic circulatory arrest with retrograde cerebral perfusion or selective antegrade cerebral perfusion. Ann Thorac Surg 2001;72:72-9. [PubMed]
- Svensson LG, Nadolny EM, Penney DL, et al. Prospective randomized neurocognitive and S-100 study of hypothermic circulatory arrest, retrograde brain perfusion, and antegrade brain perfusion for aortic arch operations. Ann Thorac Surg 2001;71:1905-12. [PubMed]
- Reich DL, Uysal S, Ergin MA, et al. Retrograde cerebral perfusion as a method of neuroprotection during thoracic aortic surgery. Ann Thorac Surg 2001;72:1774-82. [PubMed]
- Barnard J, Dunning J, Grossebner M, et al. In aortic arch surgery is there any benefit in using antegrade cerebral perfusion or retrograde cerebral perfusion as an adjunct to hypothermic circulatory arrest? Interact Cardiovasc Thorac Surg 2004;3:621-30. [PubMed]
- Usui A, Miyata H, Ueda Y, et al. Risk-adjusted and case-matched comparative study between antegrade and retrograde cerebral perfusion during aortic arch surgery: based on the Japan Adult Cardiovascular Surgery Database: the Japan Cardiovascular Surgery Database Organization. Gen Thorac Cardiovasc Surg 2012;60:132-9. [PubMed]
- Estrera AL, Garami Z, Miller CC 3rd, et al. Determination of cerebral blood flow dynamics during retrograde cerebral perfusion using power M-mode transcranial Doppler. Ann Thorac Surg 2003;76:704-9; discussion 709-10. [PubMed]
- Estrera AL, Miller CC 3rd, Lee TY, et al. Ascending and transverse aortic arch repair: the impact of retrograde cerebral perfusion. Circulation 2008;118:S160-6. [PubMed]
- Estrera AL, Miller CC, Lee TY, et al. Integrated cerebral perfusion for hypothermic circulatory arrest during transverse aortic arch repairs. Eur J Cardiothorac Surg 2010;38:293-8. [PubMed]
- Lee TY, Safi HJ, Estrera AL. Cerebral perfusion in aortic arch surgery: antegrade, retrograde, or both? Tex Heart Inst J 2011;38:674-7. [PubMed]
- Estrera AL. Is retrograde cerebral perfusion dead? Eur J Cardiothorac Surg 2013;43:1071-2. [PubMed]





