Hybrid aortic arch repair
Introduction
Thoracic endovascular aortic repair (TEVAR) has been rapidly embraced by physicians worldwide and is now a fixed part of the cardiovascular specialist’s armamentarium to treat acute and chronic thoracic aortic pathology (1-4). Extension of the technique into the aortic arch stimulated renewed interest in subclavian-to-carotid artery transposition which was primarily developed for obliterative disease. Soon it was realized that by rerouting more than one supraaortic vessel one could treat even more thoracic aortic pathology (5). As a consequence, double arterial transposition and eventually total arch rerouting were developed (6). As a further step, replacement of the ascending aorta in patients who also had aneurysmal dilation of the same segment was developed (7). The scientific community summarizes all of these approaches with the term “hybrid technique”. Thus an entirely new approach to proximal thoracic aortic pathology was developed. This article will guide the interested reader through all currently available approaches as the authors use them in the daily clinical setting.
Indications for combined approaches
Patient selection
The hybrid technique is not intended as a replacement of conventional aortic arch surgery. Firstly, the number of patients undergoing isolated aortic arch surgery due to isolated arch pathology is very low. Secondly, isolated aortic arch replacement is a straightforward operation which is technically easier than a hybrid approach. The main aim of hybrid approaches is to serve as an alternative in patients with multisegmental thoracic aortic pathology which would otherwise require a two-step conventional approach with arch replacement in the first step, and open descending repair in the second. Usually, patients presenting with multisegmental thoracic aortic pathology originating at the level of the aortic arch have an atherosclerotic etiology, which is fundamentally different when compared to patients having aortic disease in the root and the ascending aorta. This is reflected by a very high incidence of obliterative arteriopathy especially in patients with penetrating atherosclerotic ulcers (PAU). Therefore, these patients may benefit most from these combined approaches.
Preoperative diagnostic work-up and imaging
CT angiography from the carotid arteries to the femoral arteries forms the basis of any diagnostic work-up in patients being considered for treatment. This provides most of the information needed regarding morphology of the pathology, extent of rerouting procedures as well as the diameter of access vessels. Furthermore, duplex scanning of the carotid arteries is performed to exclude haemodynamically significant narrowing of the carotid bifurcation (which is rare in this setting except in patients with PAU). Cardiac echocardiography is also performed, whereas cardiac catheterization is not part of the routine diagnostic work-up in asymptomatic patients.
Surgical approach
Left subclavian-to-left carotid artery transposition
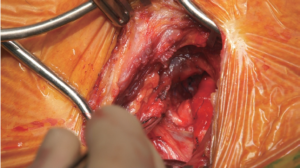
If the lesion of the distal aortic arch involves the origin of the left subclavian artery, a direct left subclavian-to-carotid artery transposition is performed through a supraclavicular incision. Both vessels are exposed between the medial and lateral clavicular insertion of the anterior scalenic muscle. Selective encircling of the left vertebral artery may help to expose the central portion of the subclavian artery. After transection of the proximal left subclavian artery, the stump is oversewn and an end-to-side anastomosis between the subclavian and the carotid artery is performed (Figure 1). This medial surgical approach helps to avoid injuries to the recurrent laryngeal and phrenic nerves, as well as to the thoracic duct. Nevertheless, careful use of electrocoagulation and ligation of all lymphatic vessels are recommended (8).
Double transposition
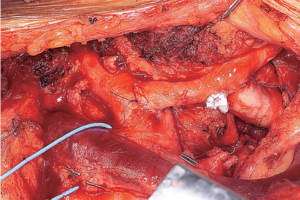
If aortic arch lesion involves the origin of the left common carotid artery, an autologous procedure to maintain cerebral perfusion may be performed. This approach was developed in 2002 (5). In the first two patients, a median sternotomy approach was used and the pericardium was opened. Skin incision was extended parallel to the left clavicle to offer sufficient access to the left subclavian artery. Later, the procedure was performed through a cranial hemisternotomy. For this procedure, it is important to provide adequate exposure of the supraaortic vessels up to their extrathoracic level to allow tension-free anastomoses. If this is not possible, an 8 mm Dacron graft is used for interposition (8).
After systemic heparinization with 80 IU per kilogram body weight, the left common carotid artery is prepared and clamped. The vessel is transected and the proximal stump is oversewn with a 4-0 Prolene running suture (Ethicon, Inc, Somerville, NJ). Thereafter, the innominate artery is clamped tangentially and a longitudinal incision is performed. A side-to-end anastomosis is then performed. Arterial pressure is assessed via either the right radial artery or both radial arteries. The advantage of the right radial artery is that any significant change in blood pressure during clamping of the innominate artery can easily be detected and corrected with more appropriate positioning of the tangential clamp. Careful flushing and de-airing are performed before antegrade blood flow is restored to the cerebral circulation. An analogous procedure is then performed between the left subclavian artery and the already transposed left common carotid artery (Figure 2). The main advantages of this approach are that it is less invasive and avoids the use of prosthetic material for the transposition of the arch vessels, which eliminates the thrombogenic risk (8). Nevertheless, several authors have described excellent results using prosthetic material in such situations (9,10).
Total arch rerouting
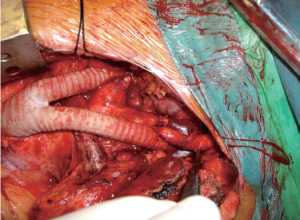
If the aortic arch lesion involves the origin of the innominate artery, using the autologous vessels alone is not suitable to assume sufficient length for an adequate proximal landing zone. Prosthetic material is necessary to re-establish perfusion of the cerebral circulation and upper extremities. In these patients, we use a reversed aortobifemoral prosthesis. The procedure is performed through median sternotomy. The pericardium is opened and after systemic heparinization with 80 IU/kg, the ascending aorta is clamped tangentially. Next, an end-to-side anastomosis is performed between the aortic side of the bifurcated prosthesis and the ascending aorta using a 4-0 Prolene running suture. The use of a xenopericardial or Teflon felt strip is an optional step to reinforce the anastomosis. Afterwards, the innominate artery is transected, the proximal stump oversewn and an end-to-end anastomosis between one distal branch of the prosthesis and the innominate artery is performed with a 5-0 Prolene running suture. Cerebral monitoring during this step of the operation is mandatory and is performed with an intraarterial catheter in the right radial artery and/or using bispectral index. The branch of the prosthesis is positioned ventrally to the innominate vein. Blood flow is restored after flushing and de-airing. Afterwards the second branch is placed either in front of or behind the innominate vein, depending on the individual anatomy, with the aim of avoiding compression and consecutive venous inflow obstruction. The left subclavian artery is clamped, transected and oversewn. An end-to-end anastomosis is performed with the second branch of the bifurcated prosthesis with a 5-0 Prolene running suture. The intraoperative situs after transposition is shown in Figure 3. In our experience a 14/7 mm or a 16/8 mm bifurcated graft is adequate for optimal size matching and provides sufficient flow, irrespective of whether Dacron or PTFE grafts are used. Finally, the left common carotid artery is transected and reimplanted into the prosthetic branch to the left subclavian artery (8).
Both options, either a simultaneous or two-step procedure - transposition or TEVAR - are feasible. The decision is based on individual patient evaluation, the local setting (such as the availability of a hybrid operating room), and the institutional experience with the type of procedure.
Involvement of the ascending aorta
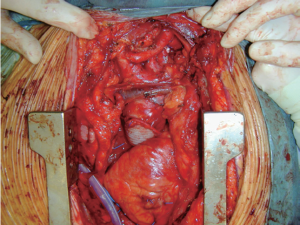
When the ascending aorta has to be replaced, due to aneurysm or extreme atherosclerosis, the right subclavian artery is approached via a subclavicular incision and cannulated for cardiopulmonary bypass, either directly or through a side branch interposition. After median sternotomy and a left cranial extension of the skin incision, the pericardium is opened; the innominate vein as well as the supraaortic branches are prepared circumferentially and encircled with silastic tapes. After systemic heparinization, CPB is instituted in normothermia and cold blood cardioplegia is used as myocardial protection. The ascending aorta is clamped just proximally to the innominate artery and resected up to the sinotubular junction. Then, the ascending aorta is replaced and any additional procedure, for instance CABG or aortic valve replacement, is performed. The aortic clamp is released, the aorta de-aired, and the patient is weaned from CPB. Afterwards, according to the individual situation, a double transposition or a total arch rerouting is done as described above. Figure 4 depicts replacement of the ascending aorta followed by double transposition. A video demonstrating the entire hybrid procedure (including TEVAR) is shown (Video 1).
Stent-graft systems
It is beyond the scope of this paper to describe in detail advantages and disadvantages of all commercially available systems. However, we would recommend the use of a device with a tip capture in the aortic arch.
Results
Outcomes of surgery
According to the recent literature, surgical results are consistently very good with regard to patency of these supraaortic rerouting procedures (9-12). However, as is the case with every new technique, problems do arise. A new pathology which arose especially following total arch rerouting was that of so-called retrograde type A aortic dissection. This seems to be a consequence of either the altered hemodynamic pattern in the proximal thoracic aorta, tangential clamp injury or a result of the compliance mismatch between the highly elastic ascending aorta and the still rigid stent-graft (13). In order to avoid this dreadful/disastrous complication, we perform prophylactic ascending aortic replacement in patients with an ascending diameter of more than 37 mm (12). Furthermore, we feel that aneurysms on the basis of chronic type B dissections should not be treated by a hybrid approach since, despite regular morphological appearance, these ascending aortas are inherently diseased.
Further complications may include injury to left laryngeal nerve as well as injury to the thoracic duct resulting in chronic lymph fistula or chylothorax. Interestingly, neurologic complications, both cerebral and spinal, are rare. This may be due to careful preservation of any collateral blood supply to the brain and to the spinal cord by maintaining antegrade perfusion of the left subclavian artery (14).
TEVAR
If a few principles are followed, the remaining risks of the procedure are low. One should respect the size of the access vessels and when in doubt should go for a larger vessel such as the common iliac artery. We also prefer to use a side graft to the native vessel as in our experience, this proved to be easier with regard to the angulation between the introduction sheath and the native anatomy (15).
Follow-up period
To date, results are stable and satisfactory (9-12). As more data are needed, it is important to include patients in a continuous follow-up protocol not only for surveillance of the treated segments but also to detect disease progression in non-treated segments as well as other pathologic processes such as colonic or lung cancer before they become clinically evident/symptomatic. Imaging therefore provides multiple benefits during follow-up in these patients.
Comments
The clinical implementation of hybrid approaches has substantially broadened the armamentarium of the cardiovascular physician in treating complex multisegmental thoracic aortic pathology. However these techniques are not intended to replace open surgery and therefore we strongly emphasize that they should only be applied in centers where the entire spectrum of treatment options is available. If this is not the case, there is a high risk that the hybrid technique would be the second best treatment option. This should be regarded also as a recommendation for the establishment of dedicated aortic centers worldwide.
Regarding technical skills, these procedures are reproducible and may be taught to any dedicated cardiovascular surgeon. It is interesting to note that the anatomic segment between the offspring of the supraaortic branches and the carotid bifurcation is largely unattended by both cardiac and vascular surgeons as the cardiac surgeon usually deals with offspring of the supraaortic branches and the vascular surgeon’s practice mostly starts at the cranial common carotid artery. These rerouting procedures have therefore stimulated interest in an anatomically neglected area (16).
Regarding technical issues, in subclavian-to-carotid transposition, we prefer a medial approach between the two insertions of the sternocleidomastoid muscle as this, in our opinion, provides optimal exposure with minimal tissue dissection. It is of utmost importance to have extensive mobilization of both the subclavian artery as well as the common carotid artery. In order to facilitate the mobilization of the subclavian artery, mobilization of the left vertebral artery is very useful.
In double transposition, the same principles regarding mobilization of vessels apply. However, due to the hemisternotomy approach, exposure is by far more convenient as is proximal vessel control. It is important to reflect the geometry of the vessels before choosing an arteriotomy site. The anastomosis between the brachiocephalic trunk and the left common carotid artery should be performed at the left lateral side of the trunk and the anastomosis between the already transposed common carotid artery and the left subclavian artery should be performed at the posterior wall of the common carotid.
In total arch rerouting, it is important to judge the distance between the ascending aorta and the posterior portion of the sternum in order to avoid complications regarding the specific distance between both structures as well as the additional proximal portion of the bifurcation prosthesis for total arch rerouting. Choosing a right lateral position of the ascending anastomosis will prevent this problem. Furthermore, it remains up to the individual anatomic situation if one or both branches of the bifurcated prosthesis are guided ventrally to the innominate vein. If any extent of arch rerouting is preceded by ascending aortic replacement, we do use right axillary artery cannulation for arterial return and do clamp the proximal arch at the level of the offspring of the brachiocephalic trunk. This is usually sufficient to exclude the entire ascending pathology whereby normothermic total arch repair can be performed.
Finally, a short word regarding the choice of the prosthesis. This article is not meant as a recommendation for one or the other product but we feel that it is important to use a device with a tip capture in the aortic arch as the deployment is by far more exact than with other strategies (17).
Outlook into the future
As the field is rapidly evolving, the next conceptual step is the implementation of branched devices, which are in fact already available. It might well be that these branched devices will have to be combined with rerouting procedures, since a fully trifurcated endovascular arch repair is still a long way off. We are very excited regarding current developments and will aim at using them diligently. However, we do hope that future developments will subscribe to the principle of “indication seeking technology” and not vice versa.
In summary, the broad implementation of supraaortic rerouting procedures to enable TEVAR within the aortic arch have broadened the armamentarium of the cardiovascular specialist in treating these complex multisegmental pathologies and have thereby opened the way for treatment for many patients who would not have been suitable for complex conventional surgery. Results of current series are very satisfying and further studies will widen our understanding of the long-term performance of the hybrid technique.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Czerny M, Cejna M, Hutschala D, et al. Stent-graft placement in atherosclerotic descending thoracic aortic aneurysms: midterm results. J Endovasc Ther 2004;11:26-32. [PubMed]
- Eggebrecht H, Nienaber CA, Neuhäuser M, et al. Endovascular stent-graft placement in aortic dissection: a meta-analysis. Eur Heart J 2006;27:489-98. [PubMed]
- Schoder M, Grabenwöger M, Hölzenbein T, et al. Endovascular stent-graft repair of complicated penetrating atherosclerotic ulcers of the descending thoracic aorta. J Vasc Surg 2002;36:720-6. [PubMed]
- Stampfl P, Greitbauer M, Zimpfer D, et al. Mid-term results of conservative, conventional and endovascular treatment for acute traumatic aortic lesions. Eur J Vasc Endovasc Surg 2006;31:475-80. [PubMed]
- Czerny M, Fleck T, Zimpfer D, et al. Combined repair of an aortic arch aneurysm by sequential transposition of the supraaortic branches and consecutive endovascular stent-graft placement. J Thorac Cardiovasc Surg 2003;126:916-8. [PubMed]
- Gottardi R, Seitelberger R, Zimpfer D, et al. An alternative approach in treating an aortic arch aneurysm with an anatomic variant by supraaortic reconstruction and stent-graft placement. J Vasc Surg 2005;42:357-60. [PubMed]
- Bavaria J, Milewski RK, Baker J, et al. Classic hybrid evolving approach to distal arch aneurysms: toward the zone zero solution. J Thorac Cardiovasc Surg 2010;140:S77-80. [PubMed]
- Czerny M, Funovics M, Schoder M, et al. Transposition of the supra-aortic vessels before stent grafting the aortic arch and descending aorta. J Thorac Cardiovasc Surg 2013;145:S91-7. [PubMed]
- Weigang E, Parker J, Czerny M, et al. Endovascular aortic arch repair after aortic arch de-branching. Ann Thorac 2009;87:603-7. [PubMed]
- Schumacher H, Von Tengg-Kobligk H, Ostovic M, et al. Hybrid aortic procedures for endoluminal arch replacement in thoracic aneurysms and type B dissections. J Cardiovasc Surg(Torino) 2006;47:509-17. [PubMed]
- Gottardi R, Funovics M, Eggers N, et al. Supra-aortic transposition for combined vascular and endovascular repair of aortic arch pathology. Ann Thorac Surg 2008;86:1524-9. [PubMed]
- Czerny M, Weigang E, Sodeck G, et al. Targeting landing zone 0 by total arch rerouting and TEVAR: midterm results of a transcontinental registry. Ann Thorac Surg 2012;94:84-9. [PubMed]
- Eggebrecht H, Thompson M, Rousseau H, et al. Retrograde ascending aortic dissection during or after thoracic aortic stent graft placement: insight from the European registry on endovascular aortic repair complications. Circulation 2009;120:S276-81. [PubMed]
- Czerny M, Eggebrecht H, Sodeck G, et al. Mechanisms of symptomatic spinal cord ischemia after TEVAR: insights from the European Registry of Endovascular Aortic Repair Complications (EuREC). J Endovasc Ther 2012;19:37-43. [PubMed]
- Czerny M, Funovics M, Sodeck G, et al. Results after thoracic endovascular aortic repair in penetrating atherosclerotic ulcers. Ann Thorac Surg 2011;92:562-6. [PubMed]
- Czerny M, Zimpfer D, Fleck T, et al. Initial results after combined repair of aortic arch aneurysms by sequential transposition of the supra-aortic branches and consecutive endovascular stent-graft placement. Ann Thorac Surg 2004;78:1256-60. [PubMed]
- Funovics M, Blum M, Langenberger H, et al. Endovascular repair of the descending aorta and the aortic arch with the Relay stent graft. Ann Thorac Surg 2009;88:637-40. [PubMed]