Type II arch hybrid debranching procedure
Introduction
The treatment of aortic arch aneurysms remains challenging (1-6). The intraoperative and the postoperative care of these patients can be quite complex, as circulatory management strategies and optimization of neurologic outcomes has to be carefully planned. Although open operative techniques have been performed with improving results over the last two decades (6), neurologic and cardiovascular complications remain significant causes of morbidity and mortality (4,5). This is especially true in patients who are at prohibitively high risk for conventional repair—such as those with older age and a high comorbidity index (5). The introduction of thoracic aortic endovascular stent grafting (TEVAR) has provided alternative surgical options in patients with complex aortic arch aneurysms, especially in the high risk population (5). Combining conventional surgical techniques with endovascular technology, the “hybrid” aortic arch repair minimizes the operation by either eliminating or significantly simplifying and shortening the arch reconstruction period, thus limiting the duration of circulatory arrest and cerebral ischemia (7-9). The arch hybrid concept entails two main principles: (I) efficient debranching of the great vessels to minimize cardiopulmonary bypass, aortic cross clamp, and circulatory arrest times, and; (II) creation of optimal proximal and distal landing zones (LZ) for TEVAR. The TEVAR component of the operation can be performed concomitantly with the open procedure, or at a later time as a retrograde approach. The hybrid arch repair is especially appealing in older patients with significant comorbidities who may not tolerate prolonged cross clamp and circulatory arrest times.
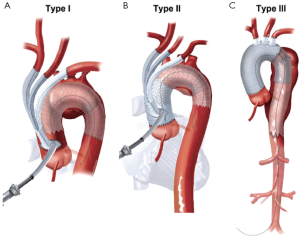
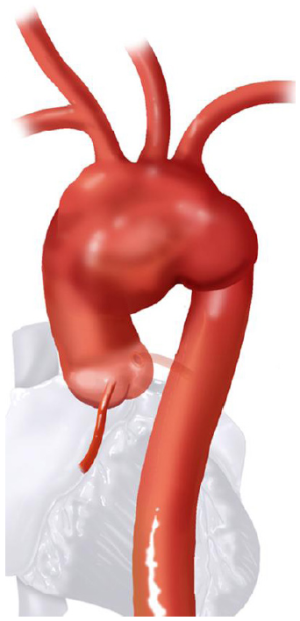
Based on the aortic arch anatomy, the required hybrid arch operative technique may vary. Therefore, hybrid arch repairs are classified into three major types, I, II and III (1-3). Construction of the required LZ for TEVAR is more extensive in type I versus II versus III. Similarly, the circulation management strategy for each type can also be increasingly complex. This report focuses on the surgical treatment options for type II aortic arch hybrid repair, where the aortic anatomy is such that the arch and ascending aorta are aneurysmal, but the descending thoracic aorta is normal. Therefore, the type II arch hybrid repair constitutes reconstruction of ascending aorta (proximal LZ for TEVAR) along with great vessel debranching, followed by antegrade or retrograde TEVAR.
The concept of arch hybrid repair for aortic arch aneurysms has been recently extended by our group for the management of complex DeBakey I aortic dissection (10). In aortic dissection patients with malperfusion syndromes, pseudocoarctation of the true lumen, or the existence of a dynamic flap, we consider the type II arch hybrid repair approach for the treatment of the aortic dissection–standard type A dissection repair with creation of a proximal LZ via a transverse hemiarch or total arch reconstruction, followed by concomitant antegrade stent grafting of the descending thoracic aorta for establishment/stabilization of true lumen flow.
Preoperative considerations
In addition to the standard work-up for open heart surgery, patients being considered for arch hybrid repair should undergo evaluation for endovascular stent grafting. This includes computed tomography angiogram (CTA) of the chest, abdomen, and pelvis, along with a programming modality to obtain three dimensional reconstruction of the entire aorta and the bilateral iliac arteries. At our institution, M2S (M2S, New Hampshire) reconstruction of the aorta is performed for all arch hybrid cases. Understanding the proximal and distal landing zones, and the ileofemoral access, is critical. There should be at least 2 cm of landing zone available both proximally and distally in order to achieve a good seal. Of note, over-extensive distal landing is not advised as it increases risk for spinal cord ischemia. In patients with previous abdominal aortic aneurysm repair, or those with long distal thoracic landing zones, spinal cord ischemia protective strategies are highly recommended. Techniques include intraoperative neuromonitoring and cerebrospinal fluid management using lumbar drain. Sensory or motor evoked potentials should be carefully monitored in the operating room. The operative plan has to be coordinated with the anesthesia and perfusion teams. These cases should be performed in hybrid operating rooms with sophisticated fixed imaging.
Operative techniques
Proximal landing zones for TEVAR and the hybrid arch repair classification scheme
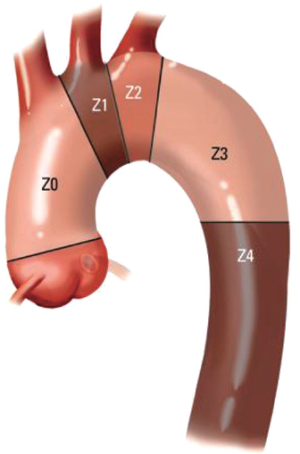
The classification scheme enables evaluation of the extent of proximal and distal LZ reconstruction and the appropriate circulatory management strategy for the operation. The hybrid arch concept entails extension of the proximal LZ to zone 0, which necessitates great vessel debranching to preserve cerebral perfusion (Figure 1). Typically TEVAR is performed with the proximal stent graft landing in zones (Z) 2 or 3. Z3 landing, which is distal to the left subclavian artery (LSCA) takeoff, is suitable for descending mid-thoracic aneurysmal disease, or some type B aortic dissections. But for proximal descending thoracic aortic aneurysms, Z2 landing, covering the LSCA is required. This may require a left common carotid (LCC) to LSCA bypass. In patients with a dominant left vertebral artery, left upper extremity ischemia, or with left internal mammary artery to left anterior descending artery coronary artery bypass grafting, the LCCA to LSCA bypass is a requirement. In these cases, we perform the bypass 2-4 days before the endovascular stent grafting. The hybrid arch concept is essentially an extension of the TEVAR proximal landing zone scheme, where typically, the stent graft is positioned in Z0.
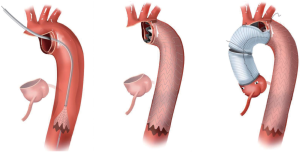
Aortic arch anatomy and the TEVAR landing zones dictate the type of arch hybrid repair (Figure 2A). In the classic, isolated aortic arch aneurysm, there are adequate proximal Z0 and distal Z3/Z4 landing zones, therefore, in a type I arch hybrid, the great vessels are debranched to enable Z0 stent grafting, followed by concomitant antegrade or delayed retrograde TEVAR. For arch aneurysm without a good proximal Z0 LZ, but an adequate Z3/Z4 distal LZ, type II arch hybrid repair is performed (Figure 2B). Therefore, the open procedure here involves not only great vessel debranching, but creation of a proximal Z0 LZ by reconstructing the ascending aorta. Thus, type II arch hybrid necessitates a period of circulatory arrest for proximal LZ reconstruction. More complex aortopathies such as mega-aorta syndrome require type III arch hybrid repair (Figure 2C). In this case, there is no proximal or distal LZ. Therefore, typically the open surgical reconstruction is more extensive, involving total arch reconstruction with elephant trunk, for concomitant or later TEVAR deployment in the descending thoracic aorta. Given the extent of coverage required for type III repair, placement of lumbar drain is highly recommended for optimization of spinal cord perfusion. This report will focus on the type II arch hybrid repair.
Type II hybrid arch
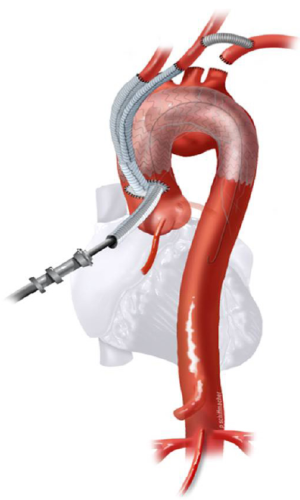
Classic anatomy mandating a type II hybrid arch approach is shown in Figure 3. In its simplest form, hybrid repair for this anatomy entails creation of an optimal Z0 proximal landing zone via ascending aorta replacement, along with great vessel debranching, followed by TEVAR. Therefore, the open surgical component involves great vessel debranching + Z0 reconstruction. The proximal extension may also require aortic root/aortic valve repair/replacement. It is important to note that the ascending aorta need not be aneurysmal to meet surgical trigger for resection; ie it does not have to be >5.5 cm. If the ascending aorta is >3.7 cm, we approach the aneurysm as a type II hybrid arch repair. In our early experience, Z0 stent graft proximal landing in ascending aorta >3.7 cm increased the risk of retrograde type A dissection. Therefore, we perform ascending aorta replacement in these patients. Unlike type I arch hybrid repair, where circulatory arrest and CPB are not required, type II reconstruction mandates a period of circulatory arrest, with adjunct cerebral perfusion strategies at moderate versus deep hypothermia. Complete knowledge of all these techniques is valuable, as the optimal circulation management strategy can be tailored to the patient’s anatomy and comorbid status.
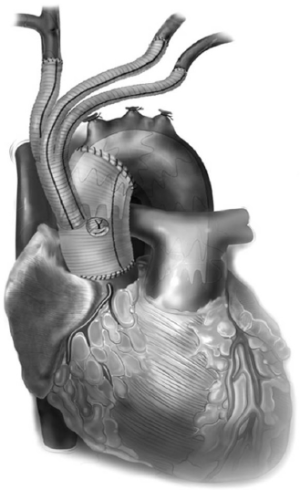
All these patients are placed on cardiopulmonary bypass (CPB). The arterial cannulation is performed via the ascending aorta, or via axillary artery cannulation through a separate right axillary incision. The latter is utilized in cases where antegrade cerebral perfusion is desired during circulatory arrest. The right atrium is cannulated for venous drainage, and if retrograde cerebral perfusion is desired during circulatory arrest, then the superior vena cava (SVC) is also cannulated and connected to the venous drainage, along with the placement of a snare around the SVC. A separate retrograde perfusion system is set up for selective retrograde cerebral flow via the SVC cannula during circulatory arrest. Most of the Z0 reconstruction and further proximal aortic root work can all be accomplished with aortic cross clamp in place. If the patient needs an aortic root or valve replacement, then it is performed first with an aortic cross clamp placed on the distal ascending aorta. If no aortic root work is required, a 4 limb branched graft with a main body straight graft is anastomosed to the aorta at the sinotubular junction. It is critical that the 4 limb branch graft lies just above the sinotubular junction, so that the proximal LZ for TEVAR is optimized. This can be done during the cooling phase on CPB. Once cooled to desired temperature, circulatory arrest is initiated and the main body graft is anastomosed to the proximal aortic arch as an open distal transverse hemiarch anastomosis. The patient is then reinitiated on CPB and the great vessel debranching is performed individually, starting with the LSCA. During transverse hemiarch anastomosis, it is not critical that an aggressive hemiarch be performed, as it will be covered by the stent graft.
During preoperative evaluation, if there is concern that LSCA exposure from the median sternotomy approach will be difficult due to lateral displacement from the arch aneurysm, a preemptive elective carotid-to-subclavian bypass (LCCA to LSCA) is a good option (Figure 4). It can also help reduce the CPB and circulatory arrest times, and may be helpful in high risk patients. This procedure is performed 2-4 days before the hybrid arch repair. The proximal LSCA is covered with the deployed stent graft in the aortic arch. Alternatively, it can be proximally ligated during the hybrid arch procedure or coiled endovascularly to prevent a type II endoleak. In some cases, the LSCA can just be sacrificed without a carotid subclavian bypass, and the stent graft may provide adequate seal without a type II endoleak. In this situation, the patient should be followed carefully for left arm ischemia, and a carotid-to-subclavian bypass can be then performed later as needed.
Retrograde cerebral perfusion (RCP) strategy for type II hybrid arch debranching
This is a good strategy in patients who would tolerate deep hypothermic circulatory arrest, have prohibitive right axillary artery anatomy, and do not require prolonged circulatory arrest time. It avoids a separate right axillary incision that would be required for antegrade cerebral perfusion. For brief circulatory arrest periods (<20 minutes), we prefer the RCP approach for type II arch hybrid repairs (Figure 5).
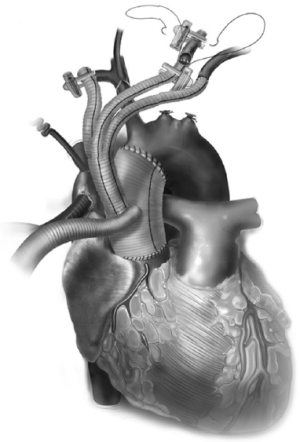
The conduct of operation is as follows. The heart is exposed in the pericardial well. The right atrial appendage is cannulated along with a right angled cannula into the superior vena cava (SVC). A snare is passed around the SVC for later control during RCP. The ascending aorta is cannulated distally and the patient is cooled for deep hypothermic circulatory arrest (DHCA), with a left ventricular vent placed via the right superior pulmonary vein. During the cooling period, the proximal ascending aortic reconstruction is done and the great vessels are dissected free. The aorta is cross clamped distally, the heart is arrested, and the proximal aortic anastomosis is performed just above the sinotubular junction using a 4 limb branched graft with a main body graft for ascending aortic replacement (Figure 5). When fashioning the main body graft for ascending aortic replacement, it is important that the branched graft portion sits right above the sinotubular junction anteriorly around 10 o’clock. This optimizes the proximal landing zone, and enables the branched grafts to lie on the side of the replaced aorta, away from the sternum. If required, any proximal aortic root work necessary can also be done during this period. Once the patient is cooled to EEG silence, DHCA is initiated, the SVC is snared down and RCP is initiated via the SVC cannula that is connected to the cardioplegia line. Typically the cerebral perfusion is done with CVP maintained <30 mmHg. The distal anastomosis is now performed as a transverse hemiarch anastomosis, which does not have to be an aggressive hemiarch, as it will be covered by the endograft.
Upon completion of the distal aortic anastomosis, the 4th limb of the branched graft can be utilized for aortic cannulation and the patient is resumed on cardiopulmonary bypass. RCP is stopped, the SVC snare is removed, and the SVC cannula is utilized for venous drainage again. Rewarming is begun, and each great vessel is anastomosed individually, with respective proximal ligation of the vessel (Figure 5). The LSCA is done first, followed by the LCCA, and then the innominate artery anastomosis. Upon completion of the great vessel debranching, the patient is weaned off cardiopulmonary bypass once the rewarming is completed.
The 4th limb of the branched graft that was utilized for arterial cannulation for cardiopulmonary bypass is now utilized for placement of a TEVAR sheath for antegrade deployment (Figure 6). For this reason, this 4th limb should be at least a 10 mm graft to facilitate placement of the sheath and the stent graft with greater ease. The proximal landing zone of the endoprosthesis is optimized so that the proximal seal occurs just above the branched graft site. The positioning can be confirmed fluoroscopically and by palpation for the proximal extent of the stent graft in the ascending aorta main body graft. Alternatively, the TEVAR component of the procedure can be performed at a later time point utilizing retrograde common femoral artery approach. If so, it is important to place radiopaque markers by the 4 limb branch graft for optimal proximal TEVAR deployment.
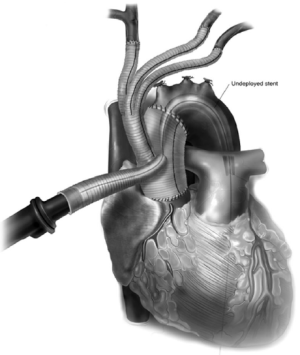
Once the stent graft is deployed, a pigtail catheter is guided up into the ascending aorta graft via the 4th limb and an arch angiogram is obtained to make sure there is proper seal proximally and distally. Alternatively, the pigtail can also be guided up from the common femoral artery in a retrograde fashion, or via a second cannula placed into the sheath, adjacent to the stent graft. If there is a type I endoleak, the stent graft can be ballooned. If the distal landing zone has a type IB endoleak, this may require an additional stent graft to be deployed into the descending thoracic aorta in an antegrade or retrograde fashion. Once completed, the 4th limb of the branched graft is ligated (Figure 7).
In patients with LSCA occlusion that can place them at higher risk for spinal cord ischemia, or renal insufficiency, a concomitant antegrade TEVAR with the open surgery may not be ideal. In these cases, patient recovery with delayed TEVAR via the retrograde approach is more suitable. One has to be aware of the interval morbidity and mortality associated with this approach. For type II arch hybrid cases, typically the patient is recovered in the hospital, and 1 to 2 weeks after the open procedure, retrograde TEVAR via the common femoral artery can be performed. Proximally, the stent graft is landed just distal to the branch graft. Distal LZ is typically in Z3/Z4. Extensive distal coverage should be avoided to minimize paraplegia risk, but it is critical that at least 2 cm of nonaneurysmal aorta is present for a good seal. Typically, it takes 2 stent grafts to cover the required length for type II repair.
Antegrade cerebral perfusion strategy
This is ideal in patients who are poor candidates for deep hypothermic circulatory arrest, and in cases where longer circulatory arrest times (>20 minutes) are anticipated. To achieve ACP, typically the right axillary artery is exposed via a separate subclavicular incision, with a proximal clamp placed on the innominate artery. The right axillary artery is exposed first (Figure 8A), and then a median sternotomy is performed and the heart and great vessels are exposed. Next, the patient is given 5,000 units of heparin (70 units/kg) and an 8 or 10 mm straight graft is anastomosed to the axillary artery (Figure 8B). The patient is then fully heparinized and the arterial cannulation is completed via the axillary artery graft. The arterial line is prepared with a Y connector—one tubing line to the axillary artery and the other for later cannulation into the branched aortic graft. Venous drainage is via right atrial cannulation and a left ventricular vent is placed. The patient is cooled to 24-28 ºC for moderate hypothermia, based on surgeon preference, during which time the proximal aortic work is typically completed. The aorta is cross clamped, the heart arrested, and the aorta is transected just above the sinotubular junction. The 4 limb branched graft is utilized and the proximal aortic anastomosis is completed. Upon cooling to the desired temperature, circulatory arrest is initiated and the patient is placed on antegrade cerebral perfusion via the axillary artery, with a soft clamp or snare tightened on the innominate artery. The distal aortic anastomosis is completed as a transverse hemiarch. Cardiopulmonary bypass can be reinitiated by increasing the arterial flow in the axillary cannula and releasing the snare. Great vessel debranching is then performed individually by ligating each vessel proximally, with an end to end anastomosis to each limb of the branch graft (Figure 9).

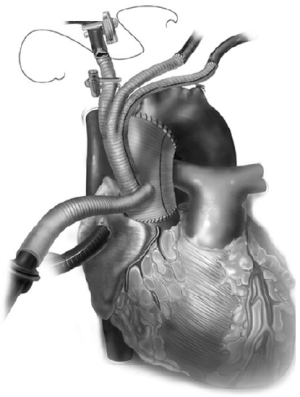
Alternatively, if the LSCA or LCCA anastomoses are difficult to perform on cardiopulmonary bypass, they can be done on circulatory arrest with ACP. Next, to complete the innominate artery anastomosis the 2nd tubing line of the arterial system is utilized for cannulation via the 4th limb of the debranching graft to restore systemic perfusion, proximal innominate artery is then ligated, and the branch graft anastomosis is completed. If the patient is warm, cardiopulmonary bypass may be terminated at this point and the 4th limb of the graft is utilized for antegrade stent graft deployment. Alternatively, if rewarming is not complete, or the heart requires longer perfusion time to improve function, the patient may be switched to the axillary artery for CPB, and the endoprosthesis can be deployed via the 4th limb in an antegrade manner.
An alternative approach to the right axillary exposure for ACP is to perform direct cannulation of the innominate artery centrally. This novel technique has been adopted by few surgeons at our institution, and the early results are promising. This approach simplifies the operation significantly. The right atrium and ascending aorta are cannulated for CPB. Patient is cooled to 24-28 ºC, during which period the proximal aortic work is completed, and the innominate artery is dissected to obtain a snare around it proximally. The innominate artery is then cannulated directly using an aortic root vent cannula, and connected to the retrograde coronary perfusion line. Circulatory arrest is initiated, the innominate artery is snared down, and ACP is started through the innominate artery root vent cannula. Upon completion of the transverse hemiarch anastomosis, CPB is restarted via the 4th limb of the branch graft, innominate artery cannula is removed along with the snare. The rest of the operation is completed as described above. This technique simplifies and reduces total incision and CPB times, provides the advantage of delivering ACP during circulatory arrest at moderate hypothermia, and avoids axillary artery exposure and its associated complications.
Type II hybrid arch approach for the treatment of complex DeBakey type I aortic dissection
Standard treatment for DeBakey I and II dissection, not involving a tear site in the aortic root or arch, entails ascending aorta + transverse hemiarch replacement with aortic valve resuspension. In DeBakey type I dissection, where a remnant dissection flap exists in the descending thoracic + abdominal aorta, complications as seen with acute type B aortic dissection can occur, such as visceral/extremity malperfusion and true lumen pseudocoarctation. In these patients, we extended the type II hybrid arch concept in treating complex DeBakey I dissection. After performing standard ascending aorta replacement, a very aggressive transverse hemiarch is tailored such that arch reconstruction is performed as a peninsula technique. Before performing the distal anastomosis, a stent graft is deployed into the true lumen of the distal arch/proximal descending thoracic aorta in an antegrade fashion under circulatory arrest (Figure 10). The graft is then gently ballooned to optimize true lumen expansion/stabilization and false lumen obliteration. The transverse hemiarch anastomosis is then performed with a straight graft, with the suture line along the lesser curve of the arch including stitches into the stent graft distally and the straight graft proximally. With this technique, nearly two-thirds to three-quarters of the aortic arch is resected, leaving primarily a portion of the greater curve involving the great vessels. Also, most of the proximal landing zone of the stent graft is fixed to the straight graft of the transverse hemiarch with this technique.
Postoperative management
Postoperative care for arch hybrid cases centers around 2 main concepts: (I) hemodynamic stability to ensure adequate organ perfusion, and, (II) spinal cord protection. Clearly, these two concepts are integrally related. Mean arterial pressure is maintained between 80 to 90 mm Hg, with higher goals (90 to 110 mmHg) as there is more extensive coverage of the descending thoracic aorta. Preoperative lumbar drain placement essential especially if the stent graft coverage goes below T6 level, or in patients with previous abdominal aortic aneurysm repair. Intrathecal pressure is maintained between 10 to 12 mm Hg.
Conclusions
Proper conduct of hybrid aortic arch surgery requires good command of both open and endovascular surgical skills. This is especially relevant as cardiac surgeons treat an increasingly aging population with higher morbidity. In the future, as thoracic endovascular technology continues to improve, there will be an increasing demand for approaches to aortic arch disease using a total endovascular platform. Aortic surgeons with endovascular skills are best trained to adopt and carefully study these newer techniques in the right patient populations. This facilitates proper evaluation of all the treatment modalities, and in designing the ideal surgical plan for a given patient.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Szeto WY, Bavaria JE, Bowen FW, et al. The hybrid total arch repair: brachiocephalic bypass and concomitant endovascular aortic arch stent graft placement. J Card Surg 2007;22:97-102; discussion 103-4. [PubMed]
- Bavaria J, Milewski RK, Baker J, et al. Classic hybrid evolving approach to distal arch aneurysms: toward the zone zero solution. J Thorac Cardiovasc Surg 2010;140:S77-80; discussion S86-91.
- Szeto WY, Bavaria JE. Hybrid repair of aortic arch aneurysms: combined open arch reconstruction and endovascular repair. Semin Thorac Cardiovasc Surg 2009;21:347-54. [PubMed]
- Kim T, Martin TD, Lee WA, et al. Evolution in the management of the total thoracic aorta. J Thorac Cardiovasc Surg 2009;137:627-34. [PubMed]
- Milewski RK, Szeto WY, Pochettino A, et al. Have hybrid procedures replaced open aortic arch reconstruction in high-risk patients? A comparative study of elective open arch debranching with endovascular stent graft placement and conventional elective open total and distal aortic arch reconstruction. J Thorac Cardiovasc Surg 2010;140:590-7. [PubMed]
- Sundt TM 3rd, Orszulak TA, Cook DJ, et al. Improving results of open arch replacement. Ann Thorac Surg 2008;86:787-96; discussion 787-96. [PubMed]
- Greenberg RK, Haddad F, Svensson L, et al. Hybrid approaches to thoracic aortic aneurysms: the role of endovascular elephant trunk completion. Circulation 2005;112:2619-26. [PubMed]
- Kouchoukos NT, Mauney MC, Masetti P, et al. Optimization of aortic arch replacement with a one-stage approach. Ann Thorac Surg 2007;83:S811-4; discussion S824-31.
- Kazui T, Yamashita K, Washiyama N, et al. Aortic arch replacement using selective cerebral perfusion. Ann Thorac Surg 2007;83:S796-8; discussion S824-31.
- Pochettino A, Brinkman NT, Moeller P, et al. Antegrade thoracic stent grafting during repair of acute DeBakey I dissection prevents development of thoracoabdominal aortic aneurysms. Ann Thorac Surg 2009;88:482-9. [PubMed]