Systematic review of robotic-assisted, totally endoscopic coronary artery bypass grafting
Introduction
The desire for a minimally invasive method of revascularizing the heart has led to the widespread use of percutaneous coronary intervention (PCI). Nonetheless, coronary artery bypass grafting (CABG) has been shown to be more efficacious in multivessel disease, with lower rates of mortality, MI and reintervention in the mid- to long-term (1). It has also been suggested that off-pump CABG can reduce morbidity associated with aortic manipulation and cardiopulmonary bypass (CPB) (2,3). As such, totally endoscopic coronary artery bypass grafting (TECABG), with patient outcomes at least as good as conventional CABG, remains the current goal.
The suggested benefits of TECABG include a reduction in surgical trauma, resulting in decreased length of hospital stay, an earlier return to normal activities and improved quality of life (4). Multiple groups have shown TECABG to be safe and reproducible, although widespread adoption of the procedure still faces major hurdles, including: expensive equipment with ongoing costs, increased technical difficulty, a steep learning curve, prolonged operating time and single-lung ventilation. As such, despite the desire for minimally invasive treatment, outcomes must be equal to or better than conventional CABG. This study aims to systematically review the intraoperative, short- and long-term patient outcomes that have been reported by the largest TECABG studies to date.
Methods
Search strategy
Electronic searches were performed in the Medline database (1946 to March 2013), Embase (1996 to March 2013), Cochrane Central Register of Controlled Trials (up to March 2013) and the Cochrane Database of Systematic Reviews (2005 to March 2013). To achieve maximum sensitivity of the search strategy and identify all studies of robotic coronary artery surgery, appropriate free text and MeSH terms were used including “coronary artery bypass” and “endoscopic” or “robotic” or “minimally invasive”. The reference lists of all retrieved articles were reviewed for further identification of potentially relevant studies.
Study selection
All studies including robotically-assisted TECABGs published in English were identified. Studies with less than 20 patients in total were excluded to avoid case-reports or articles reporting preliminary experience. When centers published duplicate or subsequent studies with accumulating numbers of patients or increased lengths of follow-up, only the most recent or complete reports were included for appraisal. The publication types of letters, editorials and reviews were excluded.
Data extraction and critical appraisal
Two investigators (M.S. and J.E.) independently reviewed each included article. Discrepancies between the two investigators were resolved by discussion and consensus with a senior investigator. The final results were reviewed by the senior investigators (M.K.W., P.G.B. and M.P.V.). Data regarding the endpoints were extracted and reported separately. Intraoperative endpoints included operating time, intraoperative exclusion rate (defined as the surgeon’s decision to not perform the procedure endoscopically due to patients factors) and intraoperative conversion rate to sternotomy or minithoracotomy (defined as the need to stop performing the procedure endoscopically). Short-term outcomes (defined as occurring within or at 1 year) included revision for bleeding (after ICU admission), all-cause mortality, stroke, new-onset atrial fibrillation (AF), renal failure, reintervention rate (defined as non-converted or excluded patients that required surgical reoperation or catheterization of the target vessel), graft patency (determined by imaging) and hospital length of stay (HLOS). Intermediate (between 1-5 years) and long-term outcomes (at or after 5 years) included cumulative survival, freedom from major adverse cardiac and cerebral events (MACCE) (a composite endpoint of cardiac mortality, MI and target vessel reintervention), freedom from angina and freedom from reintervention.
Statistical analysis
Rates of events were calculated as the number of events divided by the number of treated patients with available data. Calculation of means was weighted on the proportion of patients provided by a specific study. Patients were grouped according to whether they received beating heart total endoscopic coronary artery bypass graft (BH-TECABG) vs. arrested heart total endoscopic coronary artery bypass graft (AH-TECABG), as these two approaches are sufficiently distinct in surgical technique to warrant separate evaluation.
Surgical technique
The technique used to perform TECABG with a surgical telemanipulator has been described in detail (5,6). In summary, the left lung is collapsed, a camera port is placed in the left fifth intercostal space and two instrument ports are placed in the left third and seventh intercostal spaces. The chest is insufflated, and the left internal mammary artery (LIMA) is localised and harvested using low energy electrocautery. The right internal mammary artery (RIMA) is also accessed and harvested through the ports on the left side. The anastomosis can then be performed on an arrested heart using remote access perfusion, which is achieved with bifemoral cannulation for CPB and endoaortic balloon occlusion of the ascending aorta for delivery of cardioplegia. The anastomosis technique does not differ from the open technique. Alternatively, surgical telemanipulators that include an endoscopic suction stabiliser can be used to stabilise the beating heart and perform the anastomosis without CPB. Whilst the benefits of performing conventional CABG off-pump are contentious (7,8), it is important for managing TECABG patients with contraindications to remote access perfusion (e.g., severe peripheral vascular disease). ‘Hybrid procedures’ combine PCI either before, during, or after TECABG to achieve multivessel revascularisation.
Results
Included trials
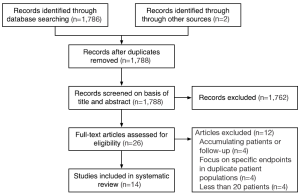
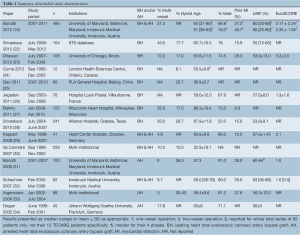
In total, 26 relevant studies were identified. Four publications were excluded as the investigators reported these results in later papers with accumulating numbers of patients and increased length of follow-up (9-12). Four more publications were excluded as the authors focused on specific endpoints in patients included in more comprehensive publications (4,13-15), although they were still considered in the discussion of results. A recent retrospective comparison of their one- and two-vessel patients by Bonatti and coworkers appeared to include patients that were reported in previous publications, so this paper was included in a separate evaluation of multivessel revascularisation (because of the appropriate format of reported endpoints) without patient duplication (16). Four studies with less than 20 patients were excluded (17-20). The study selection process is presented in Figure 1 based on the PRISMA statement (21). The 14 appraised studies allowed evaluation of 880 BH-TECABG patients, 360 AH-TECABG patients, 633 one-vessel operations and 357 two-vessel operations. A summary of the characteristics of the included studies is reported in Table 1.
Full table
Intraoperative outcomes
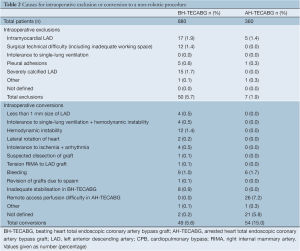
Seven studies reported causes and rates of intraoperative exclusion or conversion to CABG through sternotomy or minithoracotomy in BH-TECABG patients (22,23,25-28,30), and four studies in AHTECABG patients (30,31,33,34). Their results are summarized in Table 2. The most common causes for exclusion in both BH-TECABG and AH-TECABG were an intramyocardial left anterior descending (LAD) artery, inadequate working space (commonly reported as <3 cm), a severely calcified LAD and pleural adhesions. The most common causes for conversion in BH-TECABG were haemodynamic instability, intolerance to single-lung ventilation, bleeding and inadequate stabilization of the heart. The majority of conversions in AH-TECABG were due to difficulty with the CPB circuit, including endoaortic balloon rupture or migration and iliofemoral disease.
Full table
Almost all studies used arterial grafts exclusively. ‘Multivessel’ was defined by authors as multiple vessels grafted, not number of conduits used. Two multivessel studies reported specific revascularisation schemes (16,28). The most common one-vessel schemes were LIMA-LAD (90%), RIMA-RCA (3%) and LIMA-obtuse marginal artery (OM) (3%). The most common two-vessel schemes were LIMA-diagonal branch (Dx)/OM/circumflex artery (Cx) + RIMA-LAD (49%), LIMA-LAD-LAD jump (18%), LIMA-LAD + RIMA-Dx/OM/Cx (11%). There were only a small number of three and four vessel operations reported.
Short-term outcomes
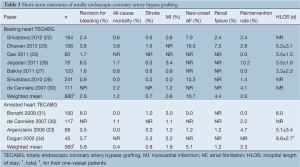
Seven studies reported short-term outcomes for BH-TECABG (22,23,25-28,30) and four studies for AH-TECABG (30,31,33,34). Their results and weighted means of all results are reported in Table 3.
Full table
Five studies reported selected endpoints separately for one- and two-vessel operations, though not all of these endpoints were the same, or were not in the same format, and thus meta-analysis was not performed. Five studies that reported operating time all demonstrated significantly increased time for two-vessel operations (16,22,23,28,34). Mean times for one-vessel were in the range of 177- 252 minutes, and mean times for two-vessel were in the range of 310-378 minutes. The earliest studies also had the longest operating times. Of the three studies that reported mortality, one had no mortality in either group, and the other two found no significant difference (16,23,28). The two studies that reported risk of intraoperative conversion rate both reported increased risk with two-vessel operations (16,28).
The short-term postoperative patency of 659 grafts performed in BH-TECABG was 98.3% and for 253 grafts in AH-TECABG was 96.4%. Patency was defined by authors as <50% stenosis and was determined using either computed tomography angiography, angiography performed during a hybrid procedure or stress ECG.
Intermediate to long-term outcomes
Four studies reported follow-up outcomes for greater than 1 year (16,24,26,29). Their results are summarized in Table 4. This included a study by Currie and coworkers that determined a graft patency of 92.7% in 12 grafts at 8±1.3 years post operation using cardiac catheterization and stress myocardial perfusion scintigraphy.
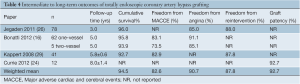
Full table
Discussion
This systematic review has demonstrated that revascularization by TECABG can achieve excellent outcomes in appropriate patients. We have highlighted the difficulties in patient selection and the most common reasons for conversion to open surgery, both in AH-TECABG and BH-TECABG.
All studies included only elective patients that were generally low-risk, and had lower rates of dyslipidemia, COPD and diabetes mellitus compared to the Society of Thoracic Surgeons (STS) database for conventional CABG patients (41.3% elective) (35). Similarly Table 1 demonstrates that most patients were relatively young and had low EuroSCOREs. Early studies limited patients to those requiring one-vessel revascularisation on an arrested heart (this includes patients who had PCI to another vessel in a hybrid procedure), which reflected the difficulty of the procedure and the limited experience of the surgeons at the time. Argenziano and colleagues performed the first prospective multicentre study between 2002-2004 that included only non-emergent one-vessel first-time LIMA-LAD revascularization patients, and had strict exclusion criteria including BMI >35, left ventricular ejection fraction (LVEF) <0.30 and other specified risk factors (33). Patients with COPD were often excluded as they have a higher risk of intolerance to the prolonged single-lung ventilation, which is supported by Lee and colleagues’ findings that patients requiring conversion had significantly lower forced vital capacity and forced expiratory volume in 1 s (36). Very obese patients (BMI >35) were often excluded, at least in part due to limited intra-thoracic space causing difficulty in maneuvering the instruments. Previous thoracic surgery resulting in the presence of adhesions was another reason for exclusion. Peripheral atherosclerotic disease, especially in the aorta and iliofemoral arteries, caused some patients to be excluded from remote access perfusion because of increased risk of stroke, arterial dissection or endoaortic balloon failure, and were thus also excluded from AH-TECABG. In later studies after the introduction of BH-TECABG, this was no longer a contraindication.
Three studies included both AH-TECABG and BH-TECABG patients (29,30,32). In two studies the operations were perform on arrested hearts first, and after gaining sufficient experience the surgeons proceeded to performing the operations on beating hearts (29,30). In the third study their decision was not detailed (32).
Multiple surgical groups who reported results for their first TECABG patients demonstrated a significant learning curve, characterized by progressively decreasing operating times, conversion rate and short term morbidity (30,31,33). Bonatti and colleagues’ learning curve stabilised in the 25-50 patient range (31). Two studies used Flex-A automatic distal anastomotic devices to reduce the technical difficulty of beating heart anastomoses and operating times (22,27). Using this device, Balkhy and colleagues achieved a short-term patency rate of 94.1% for all grafts and 98.2% for LIMA-LAD grafts (27). They reported equivalent long-term patency for the device compared to sewn anastomoses in their sternotomy CABG patients (37). These devices may play an important role in the evolution of the procedure, provided long-term results remain positive.
Overall conversion rate was higher in AH-TECABG (Table 2), mostly due to the difficulties with remote access perfusion reported by de Cannière et al. (30) and Argenziano et al. (33), including endoaortic balloon rupture or migration and poor iliofemoral condition. Conversion rates have decreased over time. Schachner and colleagues found that the only independent predictor of conversion was if the cases were performed during the surgeon’s learning curve (21% vs. 7% in surgeons who had performed ≥20 times) (13). Conversion was also less frequent in single-vessel compared to multivessel TECABs (10% vs. 25%) (13). Srivastava and colleagues found that conversion could be avoided with the careful patient selection outlined above, and techniques to solve the major causes included: ischemia management, dissection and exposure of intramyocardial target vessels, increased CO2 insufflation pressure if inadequate intrathoracic space, low tidal ventilation or continuous positive airway pressure for single-lung ventilation intolerance and freeing up adhesions (22). In addition, they suggested intraoperative conversion should not necessarily be considered a failure of TECABG, because CABG through sternotomy or minithoracotomy may be the safest approach in certain clinical situations (22).
The total number of patients undergoing BH-TECABG was higher than AH-TECABG (880 vs. 360 respectively), and the most recent studies were all BH-TECABG. This was due to surgeons switching to a beating heart method after gaining experience on an arrested heart, combined with the fact that large scale TECABG is still limited to a handful of surgical groups worldwide.
The short-term results reported by studies were heterogeneous (Table 3), and thus closer examination of these studies was worthwhile. All-cause mortality was high in two BH-TECABG studies (23,30). In Dhawan et al. 2012, four patients died of cardiac related causes, one patient had an acute infarct in a territory that was not surgically revascularized, one patient had ventricular fibrillation immediately postoperatively and was unable to be weaned off CPB due to myocardial dysfunction, one patient that was uneventfully converted to sternotomy had post-operative myocardial failure and renal failure, and one patient had complications associated with MI, bleeding and stroke. In de Cannière et al. 2007, one patient had an infarct in an area that was planned to receive PCI as part of a hybrid procedure, and one patient had acute major gastrointestinal bleeding.
Postoperative revision for bleeding was high in two studies (10,26). In Jegaden et al. 2011 this was due to thoracic wall bleeding (26). Bonatti et al. 2006 had four cases of anastomotic bleeding during their learning curve, due to the difficulty associated with performing a running suture without countertraction by an assistant (10). They reported that by modifying their protocol to include preventative repair stiches and more fibrin glue they were able to prevent further bleeding difficulties. Short-term reintervention was also high in Jegaden et al. 2011 due to problems with graft patency and post-discharge LAD bypass dysfunction detected by imaging (26).
The majority of BH-TECABG studies performed intraoperative flow measurement on their grafts (using a MediStim flow probe). In their latest study, Srivastava and colleagues achieved mean flow rates in the range of 35-45 mL/min. Few AH-TECABG studies reported intraoperative flow measurement. Short-term postoperative graft patency, as measured by computed tomography angiography or angiogram during hybrid PCI, was excellent for both BH-TECABG and AH-TECABG.
Revascularization of multivessel disease presents a significant technical challenge in TECABG. So far only a few studies have reported outcomes separately for single and multivessel TECABG patients (not including hybrid operations). Operating times reported were in the range of 3-4 hrs for one-vessel, 5-6 hrs for two-vessel and 8-9 hrs for three-vessel operations (16,23,28). Time required for multivessel operations decreased as groups overcame their learning curve, and ideal two-vessel cases have been completed in as little as 4 hours (16). Multivessel patients and increased operating time have both been significantly associated with higher rates of technical difficulties, conversion to larger incisions, longer lung separation time, more blood transfusions, longer intensive care and hospital length of stay, and higher rates of major morbidities and mortality (13,23,38,39). Despite this, in the largest multivessel study to date there was no difference in survival at 5 years, and freedom from MACCE or angina was comparable (16).
Comparison of outcomes to sternotomy CABG was approached with caution, considering that TECABG patients were elective and low-risk, the operations were performed by highly specialized groups and studies were observational in nature; thus patients were more likely to achieve better outcomes. In this review, the rate of new-onset AF after TECABG was lower than rates after sternotomy reported in the literature (35). This could be due to a reduction in trauma to the mediastinum, but could also be related to selection bias. Another suggested advantage of TECABG is an improvement in patient recovery times and quality of life, also due to the reduced surgical trauma to the thorax. In a separate study of the quality of life of AH-TECABG patients from 2002-2006 Bonaros and colleagues found that avoidance of sternotomy resulted in shorter hospital lengths of stay, lower levels of and faster improvements in postoperative pain, better general health at one month and faster return to everyday activities (by 2-3 weeks) (4). No other studies reported outcomes related to quality of life.
The recent paper by Bonatti and coworkers provided the most reliable intermediate to long-term data (16). Their 5-year results for one- and two-vessel TECABG respectively, included: cumulative survival of 95.8% and 93.9%, freedom from MACCE of 83.1% and 73.5% and freedom from angina of 91.1% and 85.1%. These results are comparable to sternotomy CABG (35), however it remains to be seen whether they are reproducible by other groups worldwide.
It could also be possible that earlier TECABG patient populations had worse long-term outcomes than more recent patients for a number of reasons, including less evolved robotic equipment (for example the addition of endoscopic stabilisers to the 2nd and 3rd generation telemanipulator enabled beating heart TECABGs), less surgeon experience, longer operating times and smaller patient numbers.
Hybrid procedures have recently attracted renewed interest. In TECABG this is in part due to the difficulty associated with operating on the RCA after placing the endoscopic ports on the left lateral thorax in order to graft the lateral vessels. Hybrid procedures also have the potential advantage of reducing operating and single-lung ventilation time, which, as previously mentioned, is associated with increased morbidity and mortality (23). Recently Bonatti and colleagues reported the largest series of 226 hybrid patients over 10 years, achieving at 5-yrs: 92.9% survival, 75.2% freedom from MACCE, 2.7% reintervention on bypass grafts and 14.2% reintervention of PCI vessels (14). Currently there are ongoing trials evaluating the potential benefits of hybrid TECABG-PCI procedures (ClinicalTrials.gov identifiers: NCT00366015; NCT01035567).
All studies in the present review were retrospective or observational reports of patients selectively chosen for TECABG. Currently there have been no randomized controlled trials that compare short- or long-term TECABG outcomes directly to median sternotomy or minithoracotomy CABG. In the majority of studies, the operations were performed at a single-center or by a single surgeon. The continually evolving field of robotics also made it difficult to assess patients together who were operated on using different generations of robotic telemanipulators and instruments. Hybrid procedures were also a confounding factor when attempting to measure the incidence of postoperative morbidities that result from TECABG.
The format, in which results were reported was heterogeneous. The field of TECABG would benefit greatly from a guideline for a systematic method of reporting results, including defined endpoints and composite measures, such as the Valve Academic Research Consortium guidelines for studies in transcatheter aortic valve implantation (40).
Significant progress in the field of TECABG has been made in the last two decades, and improvements in outcomes have continued to match robotic development and surgeon experience. There is a significant learning curve associated with the technique, which highlights the need for rigorous training and proctored experience when commencing a TECABG program. Appropriate patient selection and thorough preoperative preparation can minimize or avoid the risk of intraoperative conversion. However, these strict patient selection criteria also create a low-risk patient group, and thus comparison to sternotomy CABG is limited. Short-term outcomes have been excellent and warrant continued development of the technique. We await long-term data on the contemporary series of revascularized patients to assist in determining the efficacy of TECABG.
The next generations of cardiac surgical robots have goals aimed at reducing technical difficulty and operating times, and improving patient outcomes. Continued improvement and miniaturization of instruments, single port access platforms, automatic anastomotic connectors (41) and ‘virtual immobilization by synchronizing instrument movement with the beat of the heart (42) will help to achieve these goals.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Cao C, Manganas C, Bannon PG, et al. Drug-eluting stents versus coronary artery bypass graft surgery in left main coronary artery disease: a meta-analysis of early outcomes from randomized and nonrandomized studies. J Thorac Cardiovasc Surg 2013;145:738-47. [PubMed]
- Puskas JD, Thourani VH, Kilgo P, et al. Off-pump coronary artery bypass disproportionately benefits high-risk patients. Ann Thorac Surg 2009;88:1142-7. [PubMed]
- Edelman JJ, Yan TD, Padang R, et al. Off-pump coronary artery bypass surgery versus percutaneous coronary intervention: a meta-analysis of randomized and nonrandomized studies. Ann Thorac Surg 2010;90:1384-90. [PubMed]
- Bonaros N, Schachner T, Wiedemann D, et al. Quality of life improvement after robotically assisted coronary artery bypass grafting. Cardiology 2009;114:59-66. [PubMed]
- Bonatti J, Schachner T, Bernecker O, et al. Robotic totally endoscopic coronary artery bypass: program development and learning curve issues. J Thorac Cardiovasc Surg 2004;127:504-10. [PubMed]
- Bonatti J, Schachner T, Bonaros N, et al. Robotically assisted totally endoscopic coronary bypass surgery. Circulation 2011;124:236-44. [PubMed]
- Edelman JJ, Yan TD, Bannon PG, et al. Coronary artery bypass grafting with and without manipulation of the ascending aorta--a meta-analysis. Heart Lung Circ 2011;20:318-24. [PubMed]
- Lamy A, Devereaux PJ, Dorairaj P, et al. Effects of Off-Pump and On-Pump Coronary-Artery Bypass Grafting at 1 Year. N Engl J Med 2013;368:1179-88. [PubMed]
- Srivastava S, Gadasalli S, Agusala M, et al. Robotically Assisted Beating Heart Totally Endoscopic Coronary Artery Bypass (TECAB). Is There a Future? Innovations (Phila) 2008;3:52-8. [PubMed]
- Bonatti J, Schachner T, Bonaros N, et al. Technical challenges in totally endoscopic robotic coronary artery bypass grafting. J Thorac Cardiovasc Surg 2006;131:146-53. [PubMed]
- Gao C-Q, Yang M, Wang G, et al. Minimally invasive robotic coronary bypass on the beating heart using da Vinci S system. Zhonghua Wai Ke Za Zhi 2009;47:570-3. [PubMed]
- Gao C, Yang M, Wu Y, et al. Hybrid coronary revascularization by endoscopic robotic coronary artery bypass grafting on beating heart and stent placement. Ann Thorac Surg 2009;87:737-41. [PubMed]
- Schachner T, Bonaros N, Wiedemann D, et al. Predictors, causes, and consequences of conversions in robotically enhanced totally endoscopic coronary artery bypass graft surgery. Ann Thorac Surg 2011;91:647-53. [PubMed]
- Bonatti JO, Zimrin D, Lehr EJ, et al. Hybrid coronary revascularization using robotic totally endoscopic surgery: perioperative outcomes and 5-year results. Ann Thorac Surg 2012;94:1920-6. [PubMed]
- Bonaros N, Schachner T, Lehr E, et al. Five hundred cases of robotic totally endoscopic coronary artery bypass grafting: predictors of success and safety. Ann Thorac Surg 2013;95:803-12. [PubMed]
- Bonatti J, Lehr EJ, Schachner T, et al. Robotic total endoscopic double-vessel coronary artery bypass grafting--state of procedure development. J Thorac Cardiovasc Surg 2012;144:1061-6. [PubMed]
- Loulmet D, Carpentier A, d’Attellis N, et al. Endoscopic coronary artery bypass grafting with the aid of robotic assisted instruments. J Thorac Cardiovasc Surg 1999;118:4-10. [PubMed]
- Mishra YK, Wasir H, Sharma KK, et al. Totally endoscopic coronary artery bypass surgery. Asian Cardiovasc Thorac Ann 2006;14:447-51. [PubMed]
- Loisance DY, Nakashima K, Kirsch M. Computer-assisted coronary surgery: lessons from an initial experience. Interact Cardiovasc Thorac Surg 2005;4:398-401. [PubMed]
- Boyd WD, Rayman R, Desai ND, et al. Closed-chest coronary artery bypass grafting on the beating heart with the use of a computer-enhanced surgical robotic system. J Thorac Cardiovasc Surg 2000;120:807-9. [PubMed]
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009;6:e1000100. [PubMed]
- Srivastava S, Barrera R, Quismundo S. One hundred sixty-four consecutive beating heart totally endoscopic coronary artery bypass cases without intraoperative conversion. Ann Thorac Surg 2012;94:1463-8. [PubMed]
- Dhawan R, Roberts JD, Wroblewski K, et al. Multivessel beating heart robotic myocardial revascularization increases morbidity and mortality. J Thorac Cardiovasc Surg 2012;143:1056-61. [PubMed]
- Currie ME, Romsa J, Fox SA, et al. Long-term angiographic follow-up of robotic-assisted coronary artery revascularization. Ann Thorac Surg 2012;93:1426-31. [PubMed]
- Gao C, Yang M, Wu Y, et al. Early and midterm results of totally endoscopic coronary artery bypass grafting on the beating heart. J Thorac Cardiovasc Surg 2011;142:843-9. [PubMed]
- Jegaden O, Wautot F, Sassard T, et al. Is there an optimal minimally invasive technique for left anterior descending coronary artery bypass? J Cardiothorac Surg 2011;6:37. [PubMed]
- Balkhy HH, Wann LS, Krienbring D, et al. Integrating coronary anastomotic connectors and robotics toward a totally endoscopic beating heart approach: review of 120 cases. Ann Thorac Surg 2011;92:821-7. [PubMed]
- Srivastava S, Gadasalli S, Agusala M, et al. Beating heart totally endoscopic coronary artery bypass. Ann Thorac Surg 2010;89:1873-9; discussion1879-80.
- Kappert U, Tugtekin S-M, Cichon R, Braun M, Matschke K. Robotic totally endoscopic coronary artery bypass: a word of caution implicated by a five-year follow-up. J Thorac Cardiovasc Surg 2008;135:857-62. [PubMed]
- de Cannière D, Wimmer-Greinecker G, Cichon R, et al. Feasibility, safety, and efficacy of totally endoscopic coronary artery bypass grafting: multicenter European experience. J Thorac Cardiovasc Surg 2007;134:710-6. [PubMed]
- Bonatti J, Schachner T, Bonaros N, et al. Effectiveness and safety of total endoscopic left internal mammary artery bypass graft to the left anterior descending artery. Am J Cardiol 2009;104:1684-8. [PubMed]
- Schachner T, Feuchtner GM, Bonatti J, et al. Evaluation of robotic coronary surgery with intraoperative graft angiography and postoperative multislice computed tomography. Ann Thorac Surg 2007;83:1361-7. [PubMed]
- Argenziano M, Katz M, Bonatti J, et al. Results of the prospective multicenter trial of robotically assisted totally endoscopic coronary artery bypass grafting. Ann Thorac Surg 2006;81:1666-74; discussion1674-5.
- Dogan S, Aybek T, Andressen E, et al. Totally endoscopic coronary artery bypass grafting on cardiopulmonary bypass with robotically enhanced telemanipulation: report of forty-five cases. J Thorac Cardiovasc Surg 2002;123:1125-31. [PubMed]
- ElBardissi AW, Aranki SF, Sheng S, et al. Trends in isolated coronary artery bypass grafting: an analysis of the Society of Thoracic Surgeons adult cardiac surgery database. J Thorac Cardiovasc Surg 2012;143:273-81. [PubMed]
- Lee JD, Srivastava M, Bonatti J. History and Current Status of Robotic Totally Endoscopic Coronary Artery Bypass. Circ J 2012;76:2058-65. [PubMed]
- Balkhy HH, Wann LS, Arnsdorf SE, et al. Long term patency evaluation of the cardica c-port distal anastomotic device in coronary bypass grafting: initial experience in 91 grafts. 12th Annual Scientific Meeting of the International Society for Minimally Invasive Cardiothoracic. 2009:1-2.
- Wiedemann D, Bonaros N, Schachner T, et al. Surgical problems and complex procedures: issues for operative time in robotic totally endoscopic coronary artery bypass grafting. J Thorac Cardiovasc Surg 2012;143:639-647.e2.
- Bonatti J, Schachner T, Wiedemann D, et al. Factors influencing blood transfusion requirements in robotic totally endoscopic coronary artery bypass grafting on the arrested heart. Eur J Cardiothorac Surg 2011;39:262-7. [PubMed]
- Kappetein AP, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Am Coll Cardiol 2012;60:1438-54. [PubMed]
- Suyker WJL, Borst C. Coronary connector devices: analysis of 1,469 anastomoses in 1,216 patients. Ann Thorac Surg 2008;85:1828-36. [PubMed]
- Ortmaier T, Gröger M, Boehm DH, et al. Motion estimation in beating heart surgery. IEEE Trans Biomed Eng 2005;52:1729-40. [PubMed]





