Full myocardial revascularization with bilateral internal mammary artery Y grafts
Introduction
Bilateral internal mammary artery (BIMA) grafting in coronary artery surgery has been associated with improved long term survival and freedom from late cardiac events. Tector et al. popularized the BIMA Y configuration with a report of perioperative outcomes in 287 unselected patients in 1994 (1). A subsequent 8.5-year follow-up report of an unselected group of 897 patients who underwent full myocardial revascularization purely with BIMAs as composite grafts demonstrated the safety and mid-term benefit of the procedure (2). There have been numerous other reports of excellent perioperative outcomes in smaller cohorts. Navia et al. reported excellent results in 1,447 patients in whom myocardial revascularization was performed off pump, purely with BIMA composite grafts (3). There have been no other reports of full myocardial revascularization purely with BIMA composite grafts in patient cohorts of that magnitude, with late follow up.
Patients and methods
922 patients who underwent coronary artery graft surgery under the care of a single surgeon (HSP) using BIMAs in a Y graft configuration were identified from a cardiac surgical database of prospectively collected data from October 1994 to April 2007. The names were matched against hospital and cardiology databases in western Sydney and against the New South Wales Register of Births, Deaths and Marriages. This method of follow-up has been validated with greater than 99% accuracy (4).
Patient selection
Patients undergoing coronary bypass surgery were selected for the BIMA Y configuration according to a protocol previously described (5). This included patients aged less than 66 years who had triple vessel disease and a left ventricular ejection fraction greater than 50%. Patients were excluded from selection by protocol if any of the following existed; systemic steroid therapy, a high take off posterior descending coronary artery requiring grafting, or acute coronary insufficiency. The protocol anticipated complete myocardial revascularization using the BIMA Y graft without additional conduit and without inappropriate hazard from the BIMA harvesting. Following the identification of a 4% incidence of right phrenic nerve injury associated with high right internal mammary artery (RIMA) harvesting (6), pulmonary dysfunction was considered to be a relative contraindication, and in 2001 the protocol age was increased from 66 to 70 years. Other patients less than 70 years old underwent BIMA Y grafting in accordance with the principle that it was the preferred configuration when all grafts could be completed solely with the BIMAs using a Y configuration, or when there was insufficient alternative conduit in any patient.
Operative technique
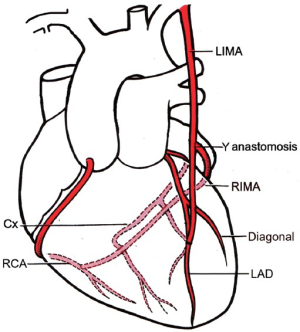
The operative technique has been described previously (5). Operations were performed through a median sternotomy with cardiopulmonary bypass and cardioplegic arrest with the exception that 3 patient underwent off pump surgery. The internal mammary arteries (IMA) were harvested using a semi-skeletonizing technique. The RIMA was harvested as a free graft with extended harvesting superiorly under the subclavian vein and inferiorly to just beyond the bifurcation. The RIMA beyond the bifurcation was not used. The first anastomosis was of the distal end of the RIMA end-to-side in parallel to its target vessel. The RIMA was then anastomosed sequentially with side-to-side perpendicular anastomoses to the left ventricular free wall vessels. It was then anastomosed end-to-side in parallel to the left IMA (LIMA). The LIMA was then anastomosed in parallel to the anterior wall vessels (Figure 1). Bypass grafts were performed to all graftable vessels with at least a 50% stenosis. For the purpose of data analysis, triple vessel disease was defined as a 50% or greater stenosis in the proximal half of the largest coronary artery in each of the three systems. Accordingly, bypass grafts were frequently performed to vessels in all three systems in patients with double vessel disease when smaller vessels had stenoses.
Post-discharge angiography
Episodes of repeat angiography were performed at the discretion of the attending cardiologist, usually for symptoms or signs suggestive of recurrent myocardial ischemia. The episodes were identified from cardiology databases and the reports reviewed. Grafts were considered to be obstructed if a graft stenosis of at least 75% existed or if there was a string sign. For statistical analysis, these were considered to be anastomotic occlusions. For patients with multiple angiograms, the last angiogram prior to coronary intervention was used for the analysis of graft obstruction. Coronary graft anastomoses (n=7) that were not part of the BIMA Y configuration were excluded from the analysis of predictors of occlusion.
Follow up
For the estimate of late survival, follow up data was collected to April 2013 (mean, 11.7 years; range, 0-18 years) and for the analysis post-discharge angiography and repeat surgical intervention data were collected to February 2008 (mean, 7.3 years; range, 0-13.3 years).
Statistical analysis
The statistical software package SPSS Version 21 was used to analyze the data. Two-tailed tests with a significance level of 5% were used throughout. Multiple logistic regression analysis with backward stepwise variable selection was used to identify the independent predictors of perioperative death. The candidate variables for inclusion were those potential risk factors associated with perioperative death at the P<0.1 level on univariate analysis. Kaplan-Meier survival curves were used to illustrate the distribution of the time from operation until death. Cox proportional hazards models were used to assess the association between survival times and various risk factors of interest and to identify the independent predictors.
For the subset of 166 patients requiring re-catheterization, the data were considered at a per graft level within patients. Multiple logistic regression analysis was used to investigate the joint effects of (I) named coronary artery; (II) anastomosis type (end to end versus side to side); (III) percentage of stenosis in coronary arteries at preoperative angiography; (IV) artery size and; (V) graded disease severity at surgery on the presence of occlusion in a graft.
The study was approved by the Western Sydney Local Health District Human Research Ethics Committee.
Results
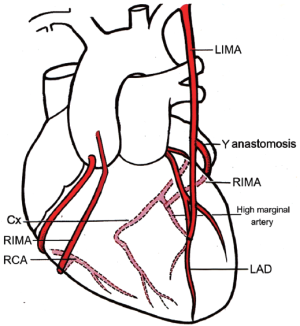
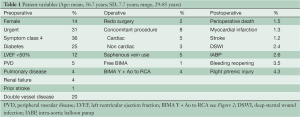
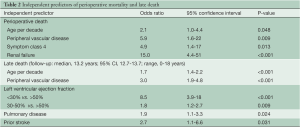
The patient cohort consisted largely of young male patients with good left ventricular function and triple vessel coronary disease. The mean additive EuroSCORE was 1.8 (interquartile range, 0-3) and the perioperative mortality was 1.5%. The major demographic, operative and perioperative variables are listed in Table 1. The mean number of IMA to coronary anastomoses was 4.1 per patient (standard deviation, 1.0; range, 2-7). Additional conduits were used in 43 patients (5%), saphenous vein in 42 and radial artery in 1 patient. These patients were included in the analysis. A modified BIMA Y graft configuration was used in 4% of patients (Figure 2). The mean length of the harvested free RIMA grafts was 18.5 cm (SD, 1.9 cm). Twenty-six patients in the study underwent subsequent redo cardiac surgery at a mean of 4 years (range, 5 months-9 years). Of the total cohort, 86% fulfilled protocol selection criteria. Non-protocol patients were predominantly those with impaired left ventricular function. Thirty-eight patients (4%) who met the protocol for BIMA Y grafting received other configurations and were not included. BIMA grafts were used in 43% of all isolated coronary artery bypass operations during the same period, and of the BIMA grafts, 87% were used in a Y configuration. Trainee surgeons performed 23% of the procedures with no significant differences in measured outcomes. The trainee operations increased from 17% to 29% after the publication of the Cleveland Clinic data on BIMA graft outcomes (7). Independent predictors of perioperative mortality and late death are presented in Table 2.
Full table
Full table
Post-discharge angiography
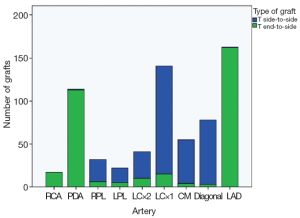
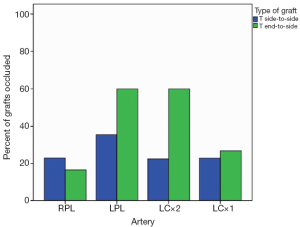
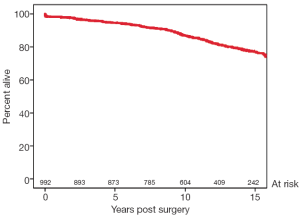
One hundred and sixty-six patients underwent post-discharge coronary angiography at a mean of 3.5 years (range, 2 weeks-13 years). In 77 patients (46%), all anastomoses were considered patent. In the 166 angiograms analyzed, the patency to the left anterior descending artery (LAD) was 91%, patency of the largest branch of the circumflex artery was 80%, and patency to that of the right coronary system was 64%. The distribution and types of grafts analyzed (end-to-side or side-to-side) are shown in Figure 3. The percentages of anastomotic occlusions for those arteries that received at least 5 of each type of anastomosis are shown in Figure 4. There was only a weak trend towards increased anastomotic occlusion for end to side anastomoses versus side to side anastomoses [odds ratio (OR), 1.7; 95% confidence interval (CI), 0.80-3.4; P=0.18] by logistic regression analysis accounting for named artery, percent stenosis of native artery at preoperative angiography, subjective grading of severity and extent of disease in the native artery at surgery, and size of artery. The interaction between the type of anastomosis and the coronary stenosis in predicting anastomotic occlusion was not significant (P=0.46) (Figure 4). Anastomoses to all named arteries except the diagonal artery had a significantly higher probability of occlusion than those to the LAD. The severity of coronary artery stenosis inversely predicted anastomotic occlusion. For end-to-side anastomoses the occlusion probability per 10% increase in native coronary stenosis severity above 50% was OR=0.75 (95% CI, 0.61-0.92; P=0.005) and for side-to-side anastomoses it was OR=0.83 (95% CI, 0.67-1.0; P=0.088). Long-term survival is shown in Figure 5. Figure 6 shows the relationship between the preoperative coronary stenosis severity and risk of anastomotic occlusion.

Discussion
LIMA versus BIMA or other
Since the Cleveland Clinic data showed that “two internal thoracic arteries are better than one” (7) for patients undergoing coronary artery surgery, there have been numerous reports from other centers showing similar benefits (8). The proponents of conventional single IMA and saphenous vein grafting rather than BIMA grafting require that the margin of benefit of BIMA surgery be sufficient to offset the cost of increased operating time and a higher rate of sternal wound complications. For surgeons who contemplate a change from conventional surgery, they not only face a learning curve associated with the change but also the dilemma of the choice between various arterial conduits and configurations.
The place of the radial artery as the second conduit is also debated. The RAPCO randomized trial comparing the radial artery to saphenous vein for the second conduit in patients aged over 70 years is not complete but has not shown any differences in clinical outcomes or graft patencies at 5 years (9). In that trial the radial artery was anastomosed to the ascending aorta. A similar randomized trial in the same age group comparing conventional bypass surgery against total arterial revascularization with composite RIMA or radial artery grafts from the LIMA showed a benefit for arterial grafting with improved graft patency and clinical outcomes (10). The Radial Artery Patency Study also showed improved radial artery patency relative to that of saphenous vein (11).
The other component of the RAPCO study compared the RIMA and the radial artery as the second conduit in patients aged less than 70 years (9). Again, both were anastomosed proximally to the ascending aorta and composite grafts were not used. At a mean follow up of 5.5 years, protocol angiography demonstrated similar patency between radial artery and free RIMA grafts (P=0.28). Ruttmann et al. used propensity matching to compare outcomes for the use of the RIMA against the radial artery as the second conduit and found that the RIMA provided benefit for both for clinical events and survival (12). Tatoulis et al. examined 1,408 symptom-driven postoperative angiograms for assessment of late IMA and radial artery graft patency. The RIMAs and radial arteries had equivalent patency rates for the circumflex and right coronary artery territories (13). For Australian surgeons, it is of note that the government remuneration is greater for a single IMA and radial artery grafting than for BIMA grafting.
In situ or free RIMA
Detachment of the RIMA for use as a free graft allows it to provide more extensive myocardial revascularization. Shah et al. showed improved graft patency to the right coronary system for free RIMA grafts over in situ grafts (14) and, also from Melbourne, Tatoulis et al. reported excellent results in 5,766 patients who underwent BIMA grafting using almost equal numbers of free and in situ RIMAs. The analysis of 991 symptom-driven angiograms showed the patency for each was similar except for grafts to the right coronary artery where the free RIMA was superior (15).
A randomized trial with mid-term angiographic follow up comparing BIMAs in Y composite or in situ configurations showed no difference in clinical outcomes or graft patency but the composite configuration achieved more arterial anastomoses (16). Calafiore et al. reported similar findings with excellent results in over 1,800 BIMA graft patients (17). Fukui et al. performed angiographic follow up to compare the outcomes of in situ to free RIMA grafts in a variety of configurations and found no difference in clinical outcomes or graft patency except when the in situ RIMA was used as the inflow for other grafts, usually with an end to end anastomosis (18).
When the free RIMA graft is applied to the circumflex system it is commonly used as part of a composite graft from the LIMA. The reasons for this preference are multiple and include the following: the composite anastomosis is ideally matched and avoids the problems of IMA anastomoses to the left side of the aorta (19), the aortic “no touch” technique reduces the risk of stroke and is particularly useful in off pump surgery, and a greater length of RIMA is available for more extensive myocardial revascularization, perhaps avoiding the use of a third conduit.
The right coronary system revascularization
Graft patency rates to the right coronary system are lower than those to the other systems and the impact of competitive flow appears greater. Few authors have compared the options for right coronary grafting in conjunction with BIMA Y grafting to the left system. Pevni et al. retrospectively analyzed the outcomes for the use of the right gastroepiploic artery, saphenous vein or the terminal segment of the RIMA from the composite graft for revascularization of the right system. There was a trend towards more perioperative complications in the terminal RIMA group but perioperative mortality and midterm survival and freedom from angina were similar (20). A randomized trial testing similar comparisons between the saphenous vein and the right gastroepiloic artery to the right coronary system incorporated a non-randomized group of terminal RIMA grafts from a BIMA Y configuration for analysis. There were no reported differences in clinical outcomes and at three-year protocol angiography the saphenous vein graft patency was significantly greater than that of the right gastroepiploic artery and the terminal RIMA. There were 20 graft occlusions for the arterial grafts which were inversely related to the functional stenosis in the right coronary artery. No difference was found between the terminal RIMA patency and that of the right gastroepiploic artery (21).
Competitive flow
This is a study of largely protocol driven BIMA Y grafting. All reasonable coronary arteries with stenosis
If right coronary revascularization can be performed with the terminal portion of the free RIMA from the BIMA Y composite graft with outcomes equivalent to the use of an alternative conduit, that would be appropriate. However, in this study there was a 4.3% incidence of right phrenic nerve injury resulting from the extended RIMA harvesting required to gain sufficient length for optimal revascularization of triple vessel disease. As previously reported, most of the phrenic neuropraxias are reversible (6) but the question remains, whether it would be better to harvest 900 additional coronary bypass conduits or accept 43 phrenic neuropraxias. The other factor that is difficult to evaluate is the increased risk of deep sternal wound infection. The incidence is higher after BIMA harvesting compared to single IMA harvesting and it is likely that extended RIMA harvesting is worse than limited RIMA harvesting (23). If only a short segment of free RIMA is required, only a short segment should be harvested. Diabetics have a higher incidence of wound infection than non-diabetics but it may be that the management of the diabetes in the perioperative period contributes to the risk of infection as much as the disease itself (24).
The long term survival of patients in this series closely matches that of a very similar demographic cohort of 5,766 patients reported by Tatoulis et al. using non-composite BIMA grafts (15). The analysis of the 991 symptom-driven angiograms showed the IMA graft patency rates to be higher than those in the current study. However, many of those angiograms identified saphenous vein graft deterioration as the cause of symptoms. There were very few vein grafts in this study and so those patients who presented for angiography due to graft dysfunction did so with IMA graft dysfunction.
Study limitations
This is not a direct patient follow-up analysis. Data relating to the incidence of death, post-discharge angiography and late surgical re-intervention were obtained through databases. Although this method has routinely provided better than 98% accuracy when checked against direct patient follow up, there remains the potential to underestimate the incidence of late adverse events. The study cohort was largely selected by protocol and therefore no control group existed. It is difficult to compare the angiographic graft patencies in this study to those of other symptom-driven angiogram analyses due to the paucity of vein grafts in this study. Analysis of predictors of anastomotic patency did not account for within patient variables or time to angiography. The absolute numbers of side-to-side anastomoses to the LAD, diagonal, posterior descending and right coronary arteries were low and did not allow comparison of anastomotic types for these vessels.
In conclusion, the perioperative and late outcomes of full myocardial revascularization purely with BIMA Y composite grafts are similar to those using BIMAs and additional conduits in other configurations. Competitive flow in the native coronary artery reduces the anastomotic patency rate of arterial grafts but there is no clear threshold beyond which arterial grafts should not be used.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Tector AJ, Amundsen S, Schmahl TM, et al. Total revascularization with T grafts.Ann Thorac Surg 1994;57:33-8; discussion 39. [PubMed]
- Tector AJ, McDonald ML, Kress DC, et al. Purely internal thoracic artery grafts: outcomes. Ann Thorac Surg 2001;72:450-5. [PubMed]
- Navia D, Vrancic M, Piccinini F, et al. Is the second internal thoracic artery better than the radial artery in total arterial off-pump coronary artery bypass grafting? A propensity score-matched follow-up study. J Thorac Cardiovasc Surg 2013. [Epub ahead of print].[PubMed]
- Mayorchak Y, Paterson HS, Ryan JB, et al. Mammary Artery to Saphenous Vein Composite T Grafts for Coronary Artery Bypass: Late Follow-up. J Cardiovasc Surg 2013;
- Nicholson IA, Paterson HS. Modified T graft for triple-vessel disease. Ann Thorac Surg 1997;64:451-3. [PubMed]
- Deng Y, Byth K, Paterson HS. Phrenic nerve injury associated with high free right internal mammary artery harvesting. Ann Thorac Surg 2003;76:459-63. [PubMed]
- Lytle BW, Blackstone EH, Loop FD, et al. Two internal thoracic artery grafts are better than one. J Thorac Cardiovasc Surg 1999;117:855-72. [PubMed]
- Tatoulis J, Buxton BF, Fuller JA. The right internal thoracic artery: is it underutilized? Curr Opin Cardiol 2011;26:528-35. [PubMed]
- Hayward PA, Gordon IR, Hare DL, et al. Comparable patencies of the radial artery and right internal thoracic artery or saphenous vein beyond 5 years: results from the Radial Artery Patency and Clinical Outcomes trial. J Thorac Cardiovasc Surg 2010;139:60-5; discussion 65-7. [PubMed]
- Muneretto C, Bisleri G, Negri A, et al. Total arterial myocardial revascularization with composite grafts improves results of coronary surgery in elderly: a prospective randomized comparison with conventional coronary artery bypass surgery. Circulation 2003;108:II29-33. [PubMed]
- Deb S, Cohen EA, Singh SK, et al. Radial artery and saphenous vein patency more than 5 years after coronary artery bypass surgery: results from RAPS (Radial Artery Patency Study). J Am Coll Cardiol 2012;60:28-35. [PubMed]
- Ruttmann E, Fischler N, Sakic A, et al. Second internal thoracic artery versus radial artery in coronary artery bypass grafting: a long-term, propensity score-matched follow-up study. Circulation 2011;124:1321-9. [PubMed]
- Tatoulis J, Buxton BF, Fuller JA. Patencies of 2127 arterial to coronary conduits over 15 years. Ann Thorac Surg 2004;77:93-101. [PubMed]
- Shah PJ, Durairaj M, Gordon I, et al. Factors affecting patency of internal thoracic artery graft: clinical and angiographic study in 1434 symptomatic patients operated between 1982 and 2002. Eur J Cardiothorac Surg 2004;26:118-24. [PubMed]
- Tatoulis J, Buxton BF, Fuller JA. The right internal thoracic artery: the forgotten conduit--5,766 patients and 991 angiograms. Ann Thorac Surg 2011;92:9-15; discussion 15-7. [PubMed]
- Glineur D, Hanet C, Poncelet A, et al. Comparison of bilateral internal thoracic artery revascularization using in situ or Y graft configurations: a prospective randomized clinical, functional, and angiographic midterm evaluation. Circulation 2008;118:S216-21. [PubMed]
- Calafiore AM, Contini M, Vitolla G, et al. Bilateral internal thoracic artery grafting: long-term clinical and angiographic results of in situ versus Y grafts. J Thorac Cardiovasc Surg 2000;120:990-6. [PubMed]
- Fukui T, Tabata M, Manabe S, et al. Angiographic outcomes of right internal thoracic artery grafts in situ or as free grafts in coronary artery bypass grafting. J Thorac Cardiovasc Surg 2010;139:868-73. [PubMed]
- Loop FD, Lytle BW, Cosgrove DM, et al. Free (aorta-coronary) internal mammary artery graft. Late results. J Thorac Cardiovasc Surg 1986;92:827-31. [PubMed]
- Pevni D, Uretzky G, Yosef P, et al. Revascularization of the right coronary artery in bilateral internal thoracic artery grafting. Ann Thorac Surg 2005;79:564-9. [PubMed]
- Glineur D, D’hoore W, de Kerchove L, et al. Angiographic predictors of 3-year patency of bypass grafts implanted on the right coronary artery system: a prospective randomized comparison of gastroepiploic artery, saphenous vein, and right internal thoracic artery grafts. J Thorac Cardiovasc Surg 2011;142:980-8. [PubMed]
- Sabik JF 3rd, Lytle BW, Blackstone EH, et al. Does competitive flow reduce internal thoracic artery graft patency? Ann Thorac Surg 2003;76:1490-6; discussion 1497. [PubMed]
- Kaya K, Kahraman D, Cavolli R, et al. Mid-segment harvesting of right internal thoracic artery decreases sternal ischemia. Anadolu Kardiyol Derg 2009;9:47-53. [PubMed]
- Deng Y, Byth K, Paterson HS. Semi-skeletonized internal mammary artery grafts and sternal wound complications. Asian Cardiovasc Thorac Ann 2004;12:227-32. [PubMed]