Improved late survival with arterial revascularization
Introduction
Grafting the left internal mammary artery (LIMA) to the left anterior descending artery (LAD) improves survival following coronary artery bypass graft (CABG) surgery in multivessel coronary artery disease (MVCAD) (1). Survival benefit of multiple arterial (MultArt) grafting is debated, and currently performed in less than 13% of CABG operations (2).
We reviewed our results with surgical revascularization of MVCAD patients, hypothesizing that MultArt CABG would present a significant long-term survival benefit compared with conventional CABG using the LIMA to the LAD with additional saphenous vein grafting (SVG) (3).
Methods
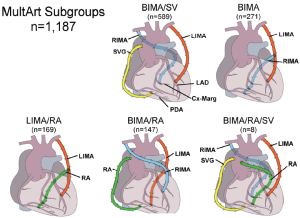
From January 1, 1993, to December 31, 2009, 8,622 consecutive MVCAD patients underwent isolated primary CABG either with LIMA and additional SVGs, the LIMA/SV group (n=7,435) or with MultArt grafts with or without the addition of SVGs, the MultArt group (n=1,187), including the following MultArt subgroups: bilateral internal mammary artery (BIMA)/SV (n=589) with the use of BIMA and SVGs, BIMA only (n=271), BIMA/radial artery (RA) (n=147), LIMA/RA (n=169), and BIMA/RA/SV (n=8) (Figure 1). There were 3 additional cases, 2 with the use of right internal mammary artery (RIMA)/RA, and 1 with the use of BIMA/gastroepiploic artery.
Indications for myocardial revascularization were based on the standard clinical and angiographic criteria. All patients were operated on through median sternotomy. Internal mammary arteries (IMAs) were harvested as pedicled or skeletonized conduits. IMAs and RAs were prepared with dilute topical solution of papaverine. Most of the operations were performed with standard cardiopulmonary bypass (CPB). Myocardial preservation during CPB involved intermittent, antegrade, or retrograde crystalloid or blood cardioplegia (28-32 °C).
The LIMA was grafted almost exclusively to the LAD and SVGs were grafted to the non-LAD vessels in LIMA/SV patients. The LIMA was also preferentially grafted to the LAD in MultArt patients, although occasionally it was used as an in situ graft to the marginal branch of the left circumflex coronary artery (LCx) with additional use of in situ RIMA to the LAD. The RIMA was grafted preferentially as an in situ graft through the transverse sinus to the marginal branch of the LCx, as a free graft in a composite-T configuration from the side of the LIMA, or, less frequently, as a free graft from the aorta to the LCx and/or to the right coronary artery (RCA) branches. The RA was used as a free graft in a composite-T configuration from the side of the LIMA or as a free graft from the aorta, to the LCx and/or to the RCA branches. SVGs were also used in MultArt subgroups preferentially to the right coronary system and less frequently to the LCx branches, diagonal or intermediate coronary vessels.
Overall, 20% of cases in the MultArt group were grafted with one artery to the LAD and second to the RCA territory, with no additional arterial grafting to the left coronary system, and 30% of RIMA grafts and 41% of RA grafts were anastomosed to the RCA territory in the MultArt group.
With approval of the Mayo Clinic Institutional Review Board and after obtaining patient consent, data were collected retrospectively by reviewing our clinical charts and computerized cardiac surgery database. Patient data were analyzed according to the Society of Thoracic Surgeons National Cardiac Surgery Database definitions. Follow-up was obtained by clinical chart review, mailed questionnaires, and the Social Security Death Index.
Descriptive statistics for categorical and continuous variables were reported as frequency and percentage, and as mean (SD), respectively. Categorical and continuous baseline variables were compared between MultArt and LIMA/SV patients by using χ2 test and 2 sample t-test or Wilcoxon rank sum test, respectively.
Logistic regression models were used to find univariate and multivariate predictors of operative mortality. Kaplan-Meier method was used to draw survival curves and calculate 5-, 10-, and 15-year survival statistics. Cox regression models were used to find the univariate and multivariate predictors of late survival and overall survival. The multivariable model considered all univariate significant variables (P<0.05) with model selection using the stepwise method. A propensity score was calculated for each patient, and 2 groups with matched propensity scores were selected. Late survival was then compared between the matched groups using Kaplan-Meier estimates and curves. All statistical tests were two-sided with the alpha level set at 0.05 for statistical significance.
Results
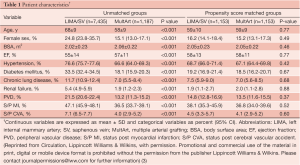
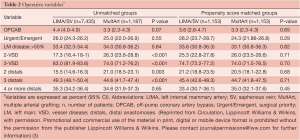
The clinical characteristics and operative variables of MultArt group and LIMA/SV group are shown in Tables 1,2, respectively. There were significant differences between the 2 unmatched groups. Aortic cross clamp time was similar in both groups (50±19 min) and bypass time was slightly longer in LIMA/SV group compared to MultArt group (85±31 and 75±30 min, respectively).
Full table
Full table
Propensity score analysis matched 1,153 patients from each group, including 97.2% of MultArt group and 15.5% of LIMA/SV group. Unadjusted operative mortality was 0.8% in MultArt group and 2.1% in LIMA/SV group (P=0.005); however, was not significantly different after multivariate adjustment or propensity score matching (P=0.996 and matched mortality 0.9% vs. 0.8%, P=0.818; respectively). In patients without operative deaths (n=8,458), follow-up ranged from 3 days to 18.3 years, with a mean of 7.6 years (SD=4.6) and median of 7.3 years. Follow-up beyond 30 days included 7,951 patients (94%).
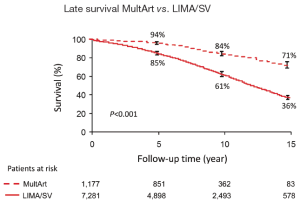
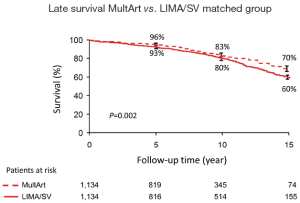
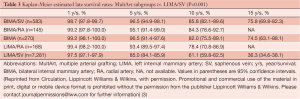
Kaplan-Meier estimated 15-year survival rates were significantly higher for patients with MultArt grafts compared to LIMA/SV group [5-, 10-, and 15-year survival rates were 95%, 84%, and 71% vs. 85%, 61%, and 36%, respectively (P<0.001) in the unmatched groups, and 96%, 83% and 70% vs. 93%, 80% and 60%, respectively (P=0.0025) in the propensity score matched groups (Figures 2,3)]. Importantly, in both figures, the cumulative survival curve of LIMA/SV group exhibits clear downsloping, and more accelerated separation of the 2 curves around 10 years.
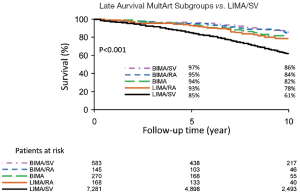
MultArt subgroups with the use of BIMA/SV and BIMA, had late survival rates of 97% and 94% at 5 years; 86% and 82% at 10 years; and 76% and 75% at 15 years, respectively (P<0.001); and subgroups with the use of BIMA/RA and LIMA/RA had late survival rates of 95% and 93% at 5 years, and 84% and 78% at 10 years, respectively (P<0.001) (Table 3 and Figure 4). BIMA/RA/SV subgroup had too few patients to be included in analysis.
Full table
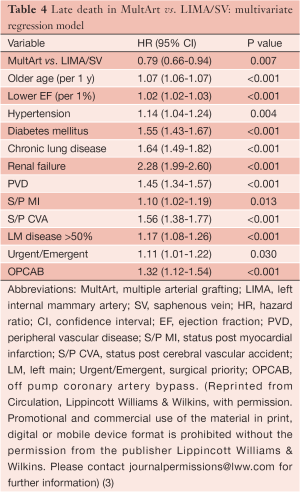
Almost all differences in patient characteristics and operative variables were identified as predicting late death in univariate Cox regression models due to the large cohort size, enabling us to include and control for all those characteristics and variables in the multivariate analysis. Older age, lower ejection fraction, hypertension, diabetes, chronic lung disease, renal failure, peripheral vascular disease, previous myocardial infarction, previous cerebral vascular accident, clinically important stenosis in the left main coronary artery, urgent/emergent surgical priority, off-pump coronary artery bypass (OPCAB), and absence of MultArt grafts were identified by multivariate Cox regression model as significant independent predictors of late death (Table 4).
Full table
The presence of MultArt grafts reduced the risk of dying by a factor of 0.79 (95% CI, 0.66-0.94) and was identified as a significant independent predictor of survival (P=0.007). Subsets of MultArt patients had significantly higher estimated rates of survival at 15 years (Table 5) (P<0.001).
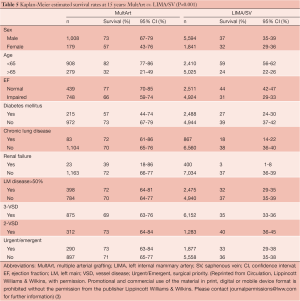
Full table
The analysis was stratified by year of surgery, before and after 2001. MultArt group had better late survival rates compared to LIMA/SV group in both eras.
Discussion
This large cohort study has shown that in primary isolated CABG performed more than 15 years ago with the use of LIMA to the LAD, bypassing the non-LAD targets with at least 1 additional arterial graft was a strong independent predictor of survival during the following 15 years. The improved survival was seen among several subsets of patients that are currently excluded at many centers from being considered to receive MultArt grafting, including patients with female sex, age older than 65 years, impaired left ventricular function, diabetes mellitus, chronic lung disease, renal failure, clinically significant left main disease, double and triple vessel disease, and those operated on with urgent/emergent surgical priority.
Long-term survival after CABG is considered to be in linear correlation with late patency of the selected conduits and grafts constructed (4). Thus, the superiority in long-term survival observed among MultArt patients, compared with LIMA/SV patients, may be related to the accelerated atherosclerosis of vein grafts with their higher rates of subsequent closure around 10 years (5). Arterial grafts possess various mechanisms that lead to increased blood flow and resistance to atherosclerosis (6).
These results, showing superior late survival with MultArt grafting, imply that the initial selection of MultArt conduits has a major influence on late survival after CABG.
The study was observational and retrospective. Thus, we cannot exclude the role of selection preferences that could contribute to improved results in the MultArt group. However, the multivariate analysis is particularly striking because of the power obtained by a very large cohort of patients, which allowed controlling for all differences between the groups. Propensity matched analysis included almost all MultArt patients and demonstrated a significant independent survival benefit associated with the use of MultArt grafting.
In conclusion, this study shows that MultArt grafting is a very important underutilized surgical tool that must be considered in all MVCAD patients, aiming to significantly improve their long-term survival.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Loop FD, Lytle BW, Cosgrove DM, et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med 1986;314:1-6. [PubMed]
- Ruttmann E, Fischler N, Sakic A, et al. Second internal thoracic artery versus radial artery in coronary artery bypass grafting: a long-term, propensity score-matched follow-up study. Circulation 2011;124:1321-9. [PubMed]
- Locker C, Schaff HV, Dearani JA, et al. Multiple arterial grafts improve late survival of patients undergoing coronary artery bypass graft surgery: analysis of 8622 patients with multivessel disease. Circulation 2012;126:1023-30. [PubMed]
- Cameron A, Davis KB, Green G, et al. Coronary bypass surgery with internal-thoracic-artery grafts: effects on survival over a 15-year period. N Engl J Med 1996;334:216-9. [PubMed]
- Lytle BW, Loop FD, Cosgrove DM, et al. Long-term (5 to 12 years) serial studies of internal mammary artery and saphenous vein coronary bypass grafts. J Thorac Cardiovasc Surg 1985;89:248-58. [PubMed]
- He GW, Liu ZG. Comparison of nitric oxide release and endothelium derived hyperpolarizing factor–mediated hyperpolarization between human radial and internal mammary arteries. Circulation 2001;104:I-344-9. [PubMed]





