Aortic arch replacement with frozen elephant trunk—when not to use it
Introduction
The most recent scientific evidence suggests the frozen elephant trunk (FET) technique plays a significant role in modern aortic arch repair operations, and is equally important for both aneurysmal disease and acute aortic dissection. Its use in extended aneurysm usually implies a therapeutic effect, aiming at complete exclusion of the diseased descending thoracic aorta. In acute aortic dissection type A (AADA), the application of FET is more prophylactic in nature, where it is primarily inserted to prevent the proximal descending thoracic aorta from late dilatation. This review will present the journey of the elephant trunk from birth to the technology currently available by dedicated clinical research (Video 1).
A short history
My predecessor in Hannover, Hans Georg Borst, published a case report in 1983 entitled “Extensive aortic replacement using ‘elephant trunk prosthesis’” (1). He did not describe the procedure as “the elephant trunk-prosthesis” but rather just “elephant trunk prosthesis,” and indeed could not have anticipated its profound development since that time. The article was published under the somewhat curious heading of “how to do it”.
In a recent article by Borst, published in the section “reflections of the pioneers” (2), he states: “The bright future of the elephant trunk technique could not be anticipated at that time. In fact, Stanley Crawford, the most experienced surgeon in the treatment of aortic pathology at that time, was quite skeptical when I first explained the elephant trunk technique to him. It did not take long, however, until his group was using this approach extensively. There probably is no method that cannot be improved.” Then, he continues. “We had performed the distal graft-to-aorta anastomosis using a continuous suture working from within the vessel, with the ‘elephant trunk’ advanced distally. It was Lars Svensson, who simplified our approach by invaginating the trunk into the arch portion of the graft, using the resulting fold for the distal graft-to-aorta anastomosis. The trunk portion of the graft was thus out of the way while performing this connection and was advanced downstream just before completing the anastomosis. The ‘FET’ technique recently described by my former coworker Matthias Karck, greatly expanded the scope of the original approach because the aortic arch and downstream aorta could now be grafted in one operative act using hybrid prostheses”.
In the current literature, there are many scientific publications on the elephant trunk technique in extended repair of thoracic aortic pathologies, the vast majority dealing with the FET (3). This bulk of recent surgical literature clearly underlines the tremendous importance of this technology for our everyday practice in aortic surgery. Nevertheless, the elephant trunk, fresh or frozen, has never gained formal medical evidence from a controlled clinical trial.
Evidence-based surgery
Surgical evidence is different from today’s standards of “evidence-based medicine” (EBM). While new pharmaceutical agents may well be introduced into clinical practice by randomized clinical trials (RCT), I am not aware of any novel surgical technique in our field entering the clinical arena by comparison to a contemporary control group. Surgical innovations are born, such as “the birth of the elephant trunk technique” (2), then modified, refined, and optimized by our surgical community. In cardiovascular surgery, the impetus for these innovations is often generated by the fate of a single patient. His surgical pathology may not be amenable for correction or would precipitate an inacceptable risk, if corrected by conventional surgical techniques. This type of progress, also called ‘disruptive innovation’, is especially true for the elephant trunk technique.
Quite often, such innovations are first published as case reports addressed to a very limited audience, where dissemination of the development is further assured by firstly discussing small patient cohorts at conferences and convincing others. Some colleagues, confronted with patients suffering from similar pathologies at their home unit, may adopt the technique to modify and optimize the original, thereby initiating an iterative process that will provide evidence for safety and efficacy. However, this evidence keeps moving, since further modifications and growing surgical routine may well improve both safety and efficacy by incremental innovation. This may be the case even decades after the initial innovative step, as seen in the elephant trunk technique.
In the setting of our daily operative routine, we find this pattern to be the most common way of generating evidence in surgery. Consider mitral valve reconstruction, the use of cardioplegia and mammary artery grafts, bilateral sequential lung transplantation, the Bentall procedure, and all other decisive innovative steps in our specialty. Such evidence is considerably different from the RCTs conducted for drugs. In the case of frozen elephant trunk, the body of evidence still increases month by month, generated by numerous center-specific publications, which also suggest new fields of indications. We should keep in mind before proposing new paradigms that the surgical theater rarely qualifies for a RCT, as best commented on by some of our international experts in the field of aortic surgery, because acute aortic dissections will never be studied in a randomized way. Thus, despite the general lack of RCTs in surgical science, we should not be accused of disregarding hypothesis driven research.
Hypotheses in surgical innovation
Non-RCT evaluation of innovation does not imply lack of a hypothesis. The initial hypothesis of Borst was that the elephant trunk lowers the risk of the second, distal aortic procedure, including the risk of lung, vessel, and nerve injury, as well as duration of descending aortic cross-clamp times. In AADA, it was hoped that the use of the elephant trunk would initiate thrombosis of the false lumen. This second hypothesis could never have been proven in a RCT, since emergency treatment is excluded from formal trials due to the issue of informed consent. The same, of course, applies to the FET. Even if we could generate a standardized population large enough for RCT, regulation would not allow us to include emergency patients. We are therefore left with single center data and registries. Such registries, despite being multi-surgeon, multi-center and multi-national by nature, unfortunately lack reproducible risk adjustment in many cases. Nevertheless, the introduction of early FETs and the development of the 4-finger hybrid graft generated new hypotheses based on the results of the conventional elephant trunk technique, a typical feature in stepwise innovation.
What are these new hypotheses to be tested and proven in ongoing and future clinical series? In aneurysmal disease, complete exclusion of the descending thoracic aneurysm can be expected, if the disease is limited to its proximal segment. Compared to conventional elephant trunks, the FET should exhibit significant improvement in morbidity and mortality, since the combined risk of interim mortality and the second intervention is abolished. In AADA, expansion of the false lumen in the proximal descending aorta is expected to result in a higher rate of false lumen thrombosis, as well as increase distal flow in cases of malperfusion due to proximal compression of the true lumen. This new approach, however, has to be closely monitored for central neurologic complications from both brain and spinal cord injury.
In terms of clinical science, surgical research in aortic arch surgery utilizing novel implants is clearly hypothesis-driven. Establishing evidence by RCTs under current guidelines will be very difficult in aneurysm surgery and impossible in acute dissections, due to the inherent complexity of the patient, the disease, and the procedure. This bears an important message for us as cardiovascular surgeons. In order to provide clinical evidence, very honest reporting of single center results is of utmost importance. Such reports should clearly stratify results according to the risk profile of the population studied, with special reference to inclusion and exclusion criteria. If we fail to do so, it may be very difficult to recommend specific surgical interventions for an individual patient in the future. For registries, an additional high level of scrutiny of reporting is required to enable the investigators to produce reliable evidence. Again, risk profiling and inclusion as well as exclusion criteria have to be thoroughly monitored to allow for meaningful recommendations based on registry data. For the time being, such registries would represent the most powerful tool to produce evidence for new procedures and devices from clinical research in cardiovascular surgery.
The technology
The original elephant trunk is nothing but a simple tube, loosely placed into the descending thoracic aorta (4), but is considered to be “the most versatile and most useful appendage on earth” by some surgeons (5). Applied by a growing number of surgeons at high numbers, this has clearly facilitated distal arch surgery (6-8).
The first elephant trunks using an aortic stent graft for distal reconstruction were hand-made (9). After the first few cases, the author contacted the retired innovator, Hans Borst, asking for an appropriate name for the new graft. He called back in less than 24 hours and suggested “FET,” and so it has been named ever since. There was growing evidence that the secondary step of descending aortic repair could be avoided in many instances. If applied in acute aortic dissection, complete thrombosis of the false lumen was observed in the majority of patients resulting in beneficial shrinking of the aortic diameter (Figure 1). Later, a commercially available hybrid graft was applied in many cases and this led to Dr. Heinz Jakob from Essen, Germany, to construct a registry for these cases. From this data (n=274), it was deduced that in more than 90% of cases operated on for AADA, there was long-term thrombosis of the false lumen in the descending aorta. In 77% of all aneurysms, complete exclusion of the diseased aortic segment could be achieved (10). By this means, further distal surgery can be avoided in selected patients, preventing morbidity and mortality in the interim phase and during reoperation.

It was Kazui who first used branched aortic grafts to facilitate supraaortic vessel anastomosis, which was initially regarded as cumbersome and time-consuming, considering that arch vessel reconstruction has to be done under circulatory arrest (11). His hypothesis was that the branched aortic arch graft would avoid pitfalls with the conventional island technique (aortic segment carrying the 3 supraaortic vessel ostia), such as bleeding during surgery and dilatation during follow-up. Our growing experience in aortic arch reconstruction initiated broader application of branched grafts worldwide and consequently, the branched FET was introduced. A fourth finger was added to enable simple access for arterial blood return from the extracorporeal circulation to the graft. The 4-finger graft now also allows for more proximal positioning of the distal suture line, following occlusion of the proximal left subclavian artery. This vessel may be reconnected after reestablishing blood flow to the heart, distal body, and the brain through the aortic prosthesis, thus shortening circulatory arrest times. Placement of the distal suture line just distal to the orifice of the left carotid artery (or even proximal) bears an indispensable advantage in all cases with lateral displacement of the left subclavian artery, which is commonly seen in aortic arch disease (12).
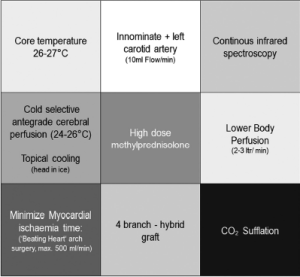
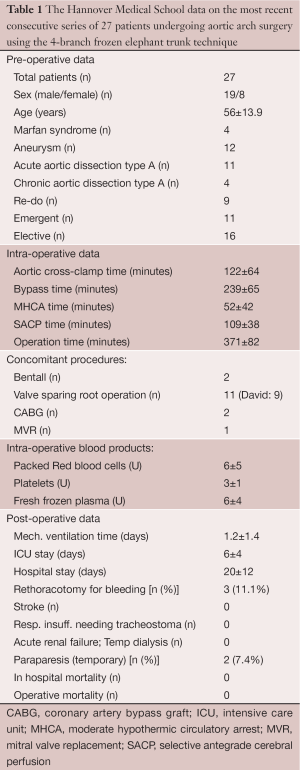
A more recent design (Thoraflex Hybrid, Vascutek) also provides a Dacron collar at the site of the distal anastomosis to allow for proper adaption of the graft diameter to the aortic diameter, which may differ substantially in aneurysmal disease. Finally, the 4-branch FET allows for early reperfusion of the heart and the distal body since no direct implantation of the supraaortic vessels into the (non-perfused) aortic arch graft is required. Early results with the use of this technique have been consecutively reported (13,14). The evolution of our refinements in perfusion technology and surgical technique has resulted in continuous modification of application of this graft, with the most recent procedural characteristics delineated in Figure 2. The results of our latest 27 patients operated on using the 4-branch FET and applying the above-mentioned procedural details were compiled by Shrestha from our group (Table 1). To our greatest satisfaction, the results indicated a substantially reduced morbidity and mortality in the complex cohort of patients with aortic arch replacement, both for aneurysmal disease and for type A acute dissections as also described by others (15,16). We now can envision outcome data for aortic arch repair comparable to those of aortic root reconstruction alone, based on these results.
Full table
In effect this type of FET, using the above-mentioned procedural details, may be advocated in all pathologies where total arch replacement and proximal descending repair is warranted or desirable. If such results become a global reality by development of evidence as described before, this approach will be the new standard of care. We would then define specific patients and procedural characteristics for application of the FET—when not to use it. This is our new hypothesis. Let us work together as a cardiovascular community to generate the evidence.
Acknowledgements
Disclosure: The author declares no conflict of interest.
References
- Borst HG, Walterbusch G, Schaps D. Extensive aortic replacement using “elephant trunk” prosthesis. Thorac Cardiovasc Surg 1983;31:37-40. [PubMed]
- Borst HG. The birth of the elephant trunk technique. J Thorac Cardiovasc Surg 2013;145:44. [PubMed]
- Tian DH, Wan B, Di Eusanio M, et al. A systematic review and meta-analysis on the safety and efficacy of the frozen elephant trunk technique in aortic arch surgery. Ann Cardiothorac Surg 2013;2:581-91.
- Karck M, Kamiya H. Progress of the treatment for extended aortic aneurysms: is the frozen elephant trunk technique the next standard in the treatment of complex aortic disease including the arch? Eur J Cardiothorac Surg 2008;33:1007-1013. [PubMed]
- Schepens MA. The most versatile and useful appendage on earth. Eur J Cardiothorac Surg 2013. [Epub ahead of print]. [PubMed]
- Hagl C, Pichlmaier M, Khaladj N. Elephant trunks in aortic surgery: fresh and frozen. J Thorac Cardiovasc Surg 2013;145:S98-102. [PubMed]
- Shrestha M, Martens A, Behrendt S, et al. Is the branched graft technique better than the en bloc technique for total aortic arch replacement? Eur J Cardiothorac Surg 2013. [Epub ahead of print]. [PubMed]
- Svensson LG, Rushing GD, Valenzuela ES, et al. Modifications, classification, and outcomes of elephant-trunk procedures. Ann Thorac Surg 2013;96:548-58. [PubMed]
- Usui A, Ueda Y, Watanabe T, et al. Clinical results of implantation of an endovascular covered stent-graft via midsternotomy for distal aortic arch aneurysm. Cardiovasc Surg 2000;8:545-9. [PubMed]
- Jakob H, Dohle DS, Piotrowski J, et al. Six-year experience with a hybrid stent graft prosthesis for extensive thoracic aortic disease: an interim balance. Eur J Cardiothorac Surg 2012;42:1018-25. [PubMed]
- Kazui T, Watanabe A, Inoue N, et al. Total aortic archgraft replacement using a prosthetic graft with three branches for acute type A aortic dissection. Kyobu Geka 1992;45:851-6; discussion 857-9. [PubMed]
- Miyamoto Y. Elephant trunk technique for hybrid aortic arch repair. Gen Thorac Cardiovasc Surg 2013. [Epub ahead of print]. [PubMed]
- Ius F, Fleissner F, Pichlmaier M, et al. Total aortic arch replacement with the frozen elephant trunk technique: 10-year follow-up single-centre experience. Eur J Cardiothorac Surg 2013. [Epub ahead of print]. [PubMed]
- Shrestha M, Pichlmaier M, Martens A, et al. Total aortic arch replacement with a novel four-branched frozen elephant trunk graft: first-in-man results. Eur J Cardiothorac Surg 2013;43:406-10. [PubMed]
- Lu S, Sun X, Hong T, et al. Modified total arch replacement using a four-branched arch graft for acute type a aortic dissection with minimal brain and spinal cord ischemic time. J Cardiovasc Surg (Torino) 2013. [Epub ahead of print]. [PubMed]
- Roselli EE, Rafael A, Soltesz EG, et al. Simplified frozen elephant trunk repair for acute DeBakey type I dissection. J Thorac Cardiovasc Surg 2013;145:S197-201. [PubMed]





