Overall Essen’s experience with the E-vita open hybrid stent graft system and evolution of the surgical technique
Introduction
The Frozen Elephant Trunk (FET) technique allows surgical access into the descending aorta via median sternotomy and is used for treatment of acute and chronic dissections as well as extensive thoracic aortic aneurysm (1,2). After the first reports from Japan and the first experience with antegrade delivery of a stent graft into the descending aorta in Europe (3-5), the development of hybrid stent graft prostheses helped to simplify and standardize the procedure (6,7). The E-vita open hybrid stent graft represents a frequently used and evaluated system by an international community of experienced centers for FET since the first implantation in 2005. Although the procedure reduces the surgical trauma compared to extensive one or two-staged aortic replacement, the FET approach requires prolonged extracorporal circulation, cerebral protection and circulatory arrest (8,9). Similar to complete arch replacement, the FET procedure introduces risks associated with neurological, cardiopulmonary, circulatory and renal complications, and hence patient selection must particularly focus on patient comorbidities. Thus, we have started to move the level of stent graft fixation proximally for easier surgical access, reduction of the ischemic times and avoidance of laryngeal nerve jeopardy. The results of our 8-year experience are presented below.
Patients and methods
Patients
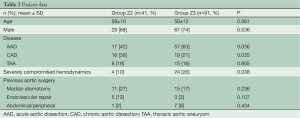
From January 2005 to July 2013, 132 patients underwent FET for acute (n=74) and chronic (n=35) aortic dissection or extensive aneurysm (n=23) in our clinic. In all cases, the E-vita open hybrid stent graft was used. During this period, changes in our surgical management have been introduced in order to increase the safety of the procedure (Table 1). The proximalization of the distal anastomosis from Zone 3 to Zone 2 was initiated in 2009 and represents our standard technique today. In order to evaluate the impact of Zone 2 anastomosis in FET procedures, patients were separated in two groups: patients with Zone 2 anastomosis (Group Z2, 41/132) and Zone 3 anastomosis (Group Z3, 91/132).

Full table
Evolution of surgical technique
E-vita open was designed and developed for atraumatic placement into the descending aorta in order to cover lesions of the aortic wall and to stabilize the true lumen in case of dissection. Bare springs were abandoned and the graft fixation was performed surgically by continuous suture with the aortic arch. The hemostatic character of the distal suture line as well as the atraumatic delivery and deployment of the stent graft encouraged the application of the procedure in acute aortic dissection (14).
Following the principles of arch replacement, the graft was fixed in Zone 3 after complete arch resection. However, the anastomosis and direct reimplantation of left subclavian artery (LSA) en-bloc or separately consumed ischemic time, especially in anatomically unfavorable situations. An additional concern of the anastomosis in Zone 3 was the preservation of laryngeal nerve integrity, especially in chronic and atherosclerotic aneurysmal cases or ruptures.
Due to strong evidence of treatment durability and minimal complications at the level of the distal anastomotic suture line during the follow-up, we began to deploy and fixate the graft in Zone 2 between the left carotid and subclavian arteries. Thus, the distal arch remains in place and the orifice of LSA is sacrificed by 2-0 polypropylene U-stitches after transection of the LSA. This moves the anastomosis towards the surgeon, facilitating and speeding up its performance and thus saving substantial amount of time.
LSA revascularization for stent graft fixation in Zone 2
LSA revascularization is performed using a 8-12 mm vascular graft with the patient on pump during cooling and prior to circulatory arrest. Alternatively, in unstable patients the anastomosis is done after aortic replacement during rewarming and cardiac reperfusion. More recently, in stable cases with unfavorable anatomy, an off-pump revascularization of the peripheral LSA/axillary artery prior to sternotomy was performed. Revascularization prior to circulatory arrest enables perfusion of the LSA and left vertebral artery via the pump after cannulation of the graft. Our management for LSA revascularization is presented in Figure 1 and illustrated in Figure 2:
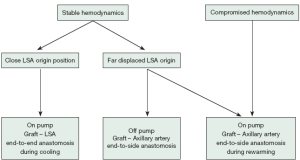
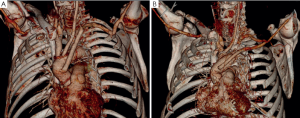
Our surgical approach in detail:
- In a hemodynamically stable situation, cannulation of the right subclavian/axillary artery (RAA) is used. After RAA preparation and median sternotomy, the arch and LSA position is evaluated.
- In the case of favorable anatomy with adequate exposure of the LSA origin, cardiopulmonary bypass and cooling of bladder temperature to 25 °C is started under continuous left ventricular venting. The brachiocephalic vein is encircled by two loops and retracted in order to enlarge the surgical field, followed by preparation of the arch and supraaortic vessels isolation by loops. The origin of LSA is clamped, transected and an end-to-end anastomosis with 5-0 propylene suture between LSA and an 8-12 mm vascular graft is performed (15). The LSA orifice is closed by 2-0 polypropylene U-stitches and the clamp is removed. After deairing, the prosthesis is cannulated and connected to a separate circuit of the heart-lung machine, which enables separate perfusion of the LSA and reimplantation in the proximal ascending/arch aortic graft during rewarming.
- When the LSA origin is difficult to navigate, extra-anatomic preparation of the left axillary artery is performed in the left deltopectoral groove. Axillary artery exposure is performed prior to cardiopulmonary bypass and encircled with a loop. After heparinization with 10,000 IU, the artery is clamped in order to expose the dorsolateral wall. The artery is opened longitudinally and a side-to-end anastomosis with an 8 cm × 15 cm vascular graft (Vascutek Ltd.) is performed. Subsequently, the graft is introduced through the 2nd intercostal space into the mediastinum, deaired and connected to a separate pump after full heparinization (400 IU/kg). Extracorporeal circulation including left arm and vertebral artery perfusion is initiated, and the LSA origin is ligated and transected during the arch operation. During rewarming, the axillary artery graft is implanted into the proximal aortic graft extra-anatomically.
- In the case of a complicated acute aortic dissection which involves compromised hemodynamics or additional dissection of the brachiocephalic trunk, the open vision direct cannulation technique of the ascending aortic true lumen is used (16). This cannulation technique requires immediate cardiac protection and arrest. Surgery starts with restoration of the proximal aorta. During the arch surgery, the LSA is ligated close to the arch and the orifice is closed by U-stiches. After completion of aortic repair and during cardiac reperfusion and rewarming, the left axillary artery is prepared as mentioned above and revascularized with an aortoaxillary bypass.
Stent graft sizing and placement
The surgical fixation of the stent graft allows avoidance of oversizing beyond 10% for stent graft stabilization. In aortic dissection, the stent graft is sized according to the diameter of the true lumen, which is evaluated preoperatively using imaging and intraoperatively using specially designed obturators (Fehling Instruments GmbH, Karlstein, Germany). This regime avoids stretching of the intimal flap, incomplete unfolding of the graft and mismatch at the anastomotic level. In aneurysmal cases minor (10-20%) oversizing is used.
The distal landing zone is evaluated by angioscopy during selective cerebral perfusion and distal circulatory arrest. The flexibility of the E-vita open and its delivery system allows deployment of the stent graft further downstream beyond the 130 mm long stent grafts, if required. Thus, distal intimal lesions can be identified angioscopically and covered. In this case, the distance between the aortic rim and the stent graft is covered by the integrated non-stented vascular prosthesis, which is pulled back gently to Zone 2 for the anastomosis. However, TH level 9 is considered as the lowest acceptable position of the stent graft end in regard to spinal cord protection.
For stent graft insertion into the descending aorta, the use of a stiff guide wire is mandatory. The wire is placed transfemorally prior to skin incision via a 6F or 8F sheath. The wire position is controlled by angiography and/or transesophageal echocardiography. In order to avoid a wire-induced aortic injury, a pig tail catheter is placed first and used as a sheath during the operation.
Arch replacement
Arch replacement is performed under selective bilateral cerebral perfusion (SACP). Circulatory arrest is initiated at bladder temperature of 25-26 °C while cerebral perfusion is maintained with 18-20 °C cold blood at a perfusion pressure of 50-60 mmHg at the cannula tip. Ice packs are placed around the head and oxygen saturation is controlled continuously by near infrared spectroscopy. After completion of the distal anastomosis at Zone 2 using a continuous 3-0 polypropylene suture with double armed visi-black needles (Ethicon, Johnson & Johnson Medical GmbH, Norderstedt, Germany) and an external Teflon® fleece, the integrated E-vita open vascular prosthesis is completely retracted and unfolded. When poor collateralization is recognized by evaluating the back blood flow through the stent graft as well as in case of circulatory arrest at more than 25 °C, a straight cannula is inserted and low body reperfusion is started. In aneurysmal cases, endoclamping by insertion of a balloon catheter into the stent graft is performed and transfemoral lower body reperfusion is started. The head vessels are reimplanted en-bloc or separately, if required. After brachiocephalic trunk reimplantation and RAA cannulation, body reperfusion and rewarming is started. If required, proximal repair is completed as well as additional procedures such as coronary artery bypass grafting or valvular repair during rewarming. In any case, cardioplegic cardiac arrest was accomplished using modified Bretschneider Solution (Custodiol, Dr. Franz Köhler Chemie GmbH, Bensheim, Germany) and was followed by implantation of the LSA vascular graft.
Statistical analysis and follow up
Data selection is performed prospectively in our institutional database for aortic surgery. The SPSS 19.0 package was used for statistical analysis. Continuous variables are presented in mean ± standard deviation (SD) and categorical variables in percent. Two-side unpaired t-test and Fischer’s-exact test were used for comparison of continuous and categorical variables, respectively. Kaplan-Meier analysis was used for survival calculation. After discharge, clinical and imaging follow-up examinations (100%) took place at 3-6 months, 12 months and annually thereafter.
Results
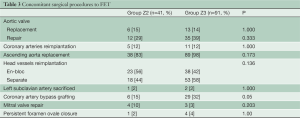
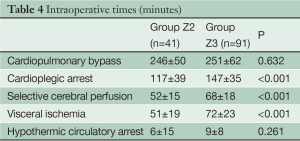
Patient characteristics are listed in Table 2. The prevalence of concomitant surgical procedures to FET was similar in both groups (Table 3), as well as the time of cardiopulmonary bypass and hypothermic circulatory arrest (Table 4). Surgical proximalization of the distal suture line from Zone 3 to Zone 2 was associated with decreased times of cardiac arrest, selective cerebral perfusion and visceral ischemia, all P<0.001. In the Z2 group, LSA revascularization was achieved by aortoaxillary bypass in 42% (17/41) and by direct aorto-subclavian bypass grafting in 56% (23/41) of patients. In one redo patient with arch/descending aorta pseudoaneurysm after previous isthmus stenosis surgery, the LSA was sacrificed.
Full table
Full table
Full table
In acute and chronic aortic dissection, complete false lumen thrombosis in the early postoperative period down to the stent graft end was 79% (26/33) in group Z2 and 84% (64/76) in group Z3 (P=0.721). Between the stent graft end and the coeliac trunk, complete false lumen exclusion and thrombosis was observed in 15% (5/33) of Z2 and 17% (13/76) of Z3 patients (P=0.921). In the aneurysm cases, full exclusion of the aneurysm without endoleak was observed in all patients of both groups.
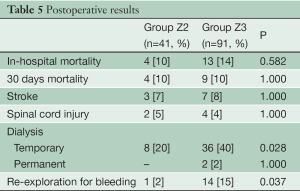
Thirty day mortality was 10% in both groups (Table 5). The prevalence of new strokes as well as paraplegia or temporary paraparesis was 8% (10/132) and 5% (6/132), respectively, and was independent from the anastomotic level (P=1.000). Anastomoses in Zone 2 were associated with less temporary dialysis (20% versus 40%, P=0.028) and re-exploration for bleeding (2% versus 15%, P=0.037) postoperatively.
Full table
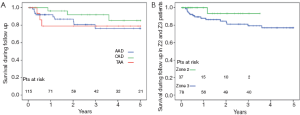
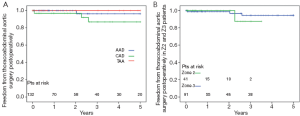
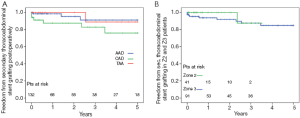
The 5-year survival rate after discharge was 76% in acute dissection, 85% in chronic dissection and 79% in aortic aneurysm patients (Figure 3). In Z2 patients, 3 year survival rate was 93% versus 79% in Z3 (P=0.135). Freedom from secondary thoracoabdominal surgery was 96% in acute dissection, 87% in chronic dissection and 100% in aneurysm patients after 5 years (Figure 4). Within the first 3 years, freedom from secondary thoracoabdominal surgery was 88% versus 94% in Z2 and Z3 patients, respectively (P=0.707). Freedom from additional stent grafting along the downstream aorta was 91% in acute dissection, 76% in chronic dissection and 89% in aneurysm patients (Figure 5). Freedom from secondary endovascular intervention was identical between both groups (88%).
Discussion
The treatment of combined ascending, arch and descending aortic disease, regardless of dissection or aneurysm, faces the limitation of inaccessibility of the descending aortic segment. Our experience with transverse thoracotomy was dismal and not compatible with our multimorbid patient population, contrasting with Kouchoukos’ findings (17). Comorbidities also represent a limitation for the classic two-stage approach, since approximately half of the patients do not return for treatment completion (18,19). The evolution of the elephant trunk technique using a self-made supported prosthesis in Japan presented a new option for antegrade treatment of the descending aorta in one stage (20,21). Between 2001-2004 we gained experience with antegrade stent grafting using grafts designed for retrograde delivery. To optimize placement and fixation relying on a classic suture line to overcome proximal endoleak problems, the E-vita open stent graft system was designed and introduced in January 2005 (7). Surgery was performed according to the classic elephant trunk technique with anastomosis in Zone 3, and appeared comparable with full arch replacement except for prolonged the ischemic times. In association with extensive aortic pathology, postoperative mortality and morbidity renders the FET as a major surgery, and not applicable to all case variations (2). However, due to the durability of the ascending arch and descending aorta repair down to the stent graft end and the low incidence of distal reoperations, a gold standard option for one-stage repair has been found (22).
To improve the safety of the FET procedure and to reduce its magnitude, several steps have been introduced, including methods for stent graft deployment control and reduction of the ischemic times (Table 1). The proximalization of the distal anastomosis from distal arch Zone 3 to Zone 2 takes into account the sacrifice of the LSA orifice and its revascularization by an extra-anatomic graft interposition. The often difficult endothoracic revascularization results in equal extracorporeal circulation times between both groups, but significantly shorter ischemic times. In some cases, transthoracic extra-anatomic bypass placement solves this dilemma elegantly. Overall, this technique enables additional antegrade perfusion of the left vertebral artery and left arm artery collaterals, which supposedly improves spinal cord protection during selective cerebral perfusion.
The interpretation of improved results with the Zone 2 anastomosis must be handled cautiously due to slight differences between the groups and the short durations of Zone 2 anastomosis cases, particularly in patients with more chronic and less acute aortic dissections. However, there is no doubt that the operative parameters could be diminished, indicated by a significant reduction of selective cerebral perfusion, cardiac and visceral ischemia times in the Z2 group. The acceleration of arch procedures seems to improve the postoperative results by reducing the prevalence of postoperative renal failure and re-explorations for bleeding. In dissection cases, moving the distal anastomosis proximal to Zone 2 had no influence on the rate of immediate false lumen thrombosis along the downstream aorta. In addition, during the 3 year follow up time, the incidence of surgical or endovascular re-interventions in the distal aorta did not increase in Z2 patients.
Debranching first with arch replacement or antegrade arch repair is reported in several variations using branched grafts combined with or without hybrid procedures (23-26), in an attempt to reduce circulatory arrest times, but at the cost of additional anastomoses for each vessel revascularization. In our experience, combination of arch replacement with en-bloc reimplantation of brachiocephalic trunk and left carotid artery when possible accelerates the duration of aortic arch procedure. Alternatively, a combination of a branched arch graft with the stent graft should be considered (27).
Our results demonstrate the feasibility and durability of one stage repair of complex thoracic aortic disease by FET with acceptable perioperative morbidity and mortality. Performing the distal anastomosis in the Z2 position facilitates the operation and shortens ischemic times, thus a reduction of complications seems warranted. Currently, this approach is our standard surgical strategy in complex thoracic aortic cases.
Acknowledgments
Disclosure: Heinz Jakob declares a conflict of interest as consultant to Jotec GmbH, Hechingen, Germany.
References
- Karck M, Kamiya H. Progress of the treatment for extended aortic aneurysms; is the frozen elephant trunk technique the next standard in the treatment of complex aortic disease including the arch? Eur J Cardiothorac Surg 2008;33:1007-13. [PubMed]
- Jakob H, Dohle DS, Piotrowski J, et al. Six-year experience with a hybrid stent graft prosthesis for extensive thoracic aortic disease: an interim balance. Eur J Cardiothorac Surg 2012;42:1018-25. [PubMed]
- Herold U, Piotrowski J, Baumgart D, et al. Endoluminal stent graft repair for acute and chronic type B aortic dissection and atherosclerotic aneurysm of the thoracic aorta: an interdisciplinary task. Eur J Cardiothorac Surg 2002;22:891-7. [PubMed]
- Fleck T, Hutschala D, Czerny M, et al. Combined surgical and endovascular treatment of acute aortic dissection type A: preliminary results. Ann Thorac Surg 2002;74:761-5; discussion 765-6. [PubMed]
- Herold U, Tsagakis K, Kamler M, et al. Change of paradigms in the surgical treatment of complex thoracic aortic disease. Herz 2006;31:434-42. [PubMed]
- Karck M, Chavan A, Hagl C, et al. The frozen elephant trunk technique: a new treatment for thoracic aortic aneurysms. J Thorac Cardiovasc Surg 2003;125:1550-3. [PubMed]
- Jakob H, Tsagakis K, Leyh R, et al. Development of an integrated stent graft-dacron prosthesis for intended one-stage repair in complex thoracic aortic disease. Herz 2005;30:766-8. [PubMed]
- Tsagakis K, Pacini D, Di Bartolomeo R, et al. Arch replacement and downstream stent grafting in complex aortic dissection: first results of an international registry. Eur J Cardiothorac Surg 2011;39:87-93; discussion 93-4. [PubMed]
- Shrestha M, Pichlmaier M, Martens A, et al. Total aortic arch replacement with a novel four-branched frozen elephant trunk graft: first-in-man results. Eur J Cardiothorac Surg 2013;43:406-10. [PubMed]
- Tsagakis K, Kamler M, Benedik J, et al. Angioscopy--a valuable tool in guiding hybrid stent grafting and decision making during type A aortic dissection surgery. Eur J Cardiothorac Surg 2010;38:507-9. [PubMed]
- Tsagakis K, Pizanis N, Baba HA, et al. Impermeability to blood of the E-vita open plus hybrid stent-graft: experimental and clinical evaluation. J Endovasc Ther 2010;17:340-8. [PubMed]
- Pacini D, Tsagakis K, Jakob H, et al. The frozen elephant trunk for the treatment of chronic dissection of the thoracic aorta: a multicenter experience. Ann Thorac Surg 2011;92:1663-70; discussion 1670.
- Tsagakis K, Dohle DS, Piotrowski J, et al. Time saving modification of arch replacement in frozen elephant trunk procedure. Thorac cardiovasc Surg 2012;60-V59.
- Jakob H, Tsagakis K, Tossios P, et al. Combining classic surgery with descending stent grafting for acute DeBakey type I dissection. Ann Thorac Surg 2008;86:95-101. [PubMed]
- Jakob H. Facilitated surgical strategy in total arch replacement and descending aorta stent grafting with the E-vita open hybrid prosthesis. Ann Cardiothorac Surg 2013;2:663-4.
- Jakob H, Tsagakis K, Szabo A, et al. Rapid and safe direct cannulation of the true lumen of the ascending aorta in acute type A aortic dissection. J Thorac Cardiovasc Surg 2007;134:244-5. [PubMed]
- Kouchoukos NT. One-stage repair of extensive thoracic aortic disease. J Thorac Cardiovasc Surg 2010;140:S150-3; discussion S185-S190.
- Etz CD, Plestis KA, Kari FA, et al. Staged repair of thoracic and thoracoabdominal aortic aneurysms using the elephant trunk technique: a consecutive series of 215 first stage and 120 complete repairs. Eur J Cardiothorac Surg 2008;34:605-14; discussion 614-5. [PubMed]
- Shrestha M, Martens A, Krüger H, et al. Total aortic arch replacement with the elephant trunk technique: single-centre 30-year results. Eur J Cardiothorac Surg 2013. [Epub ahead of print]. [PubMed]
- Kato M, Kuratani T, Kaneko M, et al. The results of total arch graft implantation with open stent-graft placement for type A aortic dissection. J Thorac Cardiovasc Surg 2002;124:531-40. [PubMed]
- Uchida N, Katayama A, Tamura K, et al. Long-term results of the frozen elephant trunk technique for extended aortic arch disease. Eur J Cardiothorac Surg 2010;37:1338-45. [PubMed]
- Jakob H, Tsagakis K. International E-vita open registry. Ann Cardiothorac Surg 2013;2:296-9. [PubMed]
- Spielvogel D, Etz CD, Silovitz D, et al. Aortic arch replacement with a trifurcated graft. Ann Thorac Surg 2007;83:S791-5; discussion S824-31.
- Shimamura K, Kuratani T, Matsumiya G, et al. Long-term results of the open stent-grafting technique for extended aortic arch disease. J Thorac Cardiovasc Surg 2008;135:1261-9. [PubMed]
- Bavaria J, Milewski RK, Baker J, et al. Classic hybrid evolving approach to distal arch aneurysms: toward the zone zero solution. J Thorac Cardiovasc Surg 2010;140:S77-80; discussion S86-91.
- Matalanis G, Koirala RS, Shi WY, et al. Branch-first aortic arch replacement with no circulatory arrest or deep hypothermia. J Thorac Cardiovasc Surg 2011;142:809-15. [PubMed]
- Shrestha M, Pichlmaier M, Martens A, et al. Total aortic arch replacement with a novel four-branched frozen elephant trunk graft: first-in-man results. Eur J Cardiothorac Surg 2013;43:406-10. [PubMed]





