Trade in the hammer for a power driver—perspectives on the frozen elephant trunk repair for aortic arch disease
To a man with a hammer everything looks like a nail.
—Mark Twain
The ‘hammer’ for patients requiring complex arch reconstruction, which involves straight circulatory arrest and a sutured repair for every anastomosis, is becoming less common. Like a versatile ‘power driver’ for which the bit can be exchanged to match the screw turned, improvement of brain protection strategies and the development of hybrid techniques have provided us the ability to offer tailored repair options for patients with complex thoracic disease involving the arch.
Hybrid repairs that involve ‘debranching’ the arch by transposing the supra-aortic vessels more proximally followed by thoracic endovascular aortic repair (TEVAR) across the curvature of the arch have demonstrated limited success. These operations have been associated with a relatively high risk for stroke and endoleaks, and do not allow for the simultaneous performance of concomitant cardiovascular procedures (1). Furthermore, delivering the stentgraft across a diseased aortic arch increases the risk of stroke due to atheroembolization (2). To control the aortic arch more safely, the use of selective brain perfusion and circulatory arrest in combination with direct antegrade delivery of a stentgraft may reduce this risk. Kato first described the use of a graft with a distal stent as a modification of the conventional elephant trunk procedure, and Karck coined the term ‘frozen elephant trunk’ (FET) to describe this approach (3,4). While many variations of how to perform this procedure have been introduced and described, the common feature is that the stentgraft is delivered into the open aorta under circulatory arrest and sutured into position.
Uchida developed a version of this technique using a stented graft sewn at the level distal to the left subclavian artery (5). Sun’s method of repair moved the suture line proximally with a fully stented device, and also included separate branch reconstruction of the arch vessels during antegrade brain perfusion (6). Specifically designed hybrid devices (E-vita, Jotec, Germany; Thoraflex, Vascutek Terumo, Scotland) are available in Europe and are currently being evaluated as part of multi-center studies (7,8). These approaches offer the security of fixation provided by direct suturing of the stentgraft proximally, and the avoidance of a second open chest incision by stentgraft expansion at the most distal point of repair.
As I have gained experience using various techniques for high-risk aortic surgery, it is clear that each has its merits and limitations. Variations of the FET operation have been the most versatile. It is possible to choose the best procedure for each patient based on the etiology of disease, the time and urgency of presentation, and the operative indications. This article describes various methods of performing the FET procedure in different situations with a focus on considerations during the planning process.
FET for degenerative aortic aneurysm
It has been shown that the risk of spinal cord injury is most closely associated with the overall extent of aorta treated (9). For this reason, we have limited use of the single stage FET technique for degenerative aneurysms to patients who only require stent grafting of the upper half of their descending aorta. If the patient requires more extensive coverage of the aorta and a prompt completion, then we delay two-stage repair by five days to six weeks depending on the urgency for completion (10). This staging process allows the perioperative inflammatory process to subside before the additional insult to spinal cord circulation associated with stent grafting.
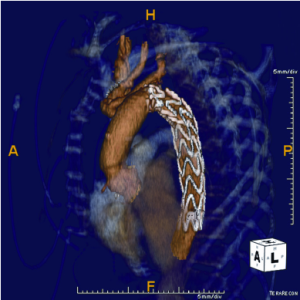
For patients with degenerative aneurysms involving the arch and more proximal descending aorta, we perform the FET reconstruction using circulatory arrest with selective antegrade brain perfusion (SABP) via right axillary artery cannulation and separate debranching of the supra-aortic vessels (11) (Figure 1). The reconstructive techniques involving separate reconstruction of the branch vessels in lieu of a Carrel patch provides a more hemostatic and versatile alternative that has been made safer with the use of SABP. Indeed, SABP is the “power driver” that has opened up the toolbox available to aortic surgeons.
In the United States, all of the currently available stentgraft devices are intended for transfemoral endovascular delivery. We have previously shown, however, that it is safe to deliver any of the commercially available devices in an antegrade fashion through the aortic arch over a wire (12). When using one of these devices for FET, it is easier to choose one with a delivery system that allows for direct visualization of the device so that the positioning is most accurate.
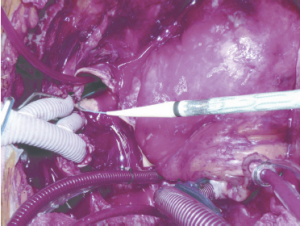
To avoid the risk of distal aortic injury from blind delivery into the open descending aorta (especially a very tortuous descending aorta), we have developed a delivery technique which first entails gaining wire and catheter access from a puncture in one of the common femoral arteries through a 5 Fr sheath. Through this previously placed catheter, through and through wire access can be achieved with a stiff wire to ensure safe delivery into the distal aorta without direct visualization (Figure 2). Jakob has described use of an endoscope to assess the device after deployment (13); however, I aborted this approach early in my experience as this may prolong the circulatory arrest period compared to using the pre-delivered wire. The wire placement may be confirmed by transesophageal echocardiography. However, for patients with complex anatomy such as severe tortuosity or chronic dissection, the use of fluoroscopic guidance either in the hybrid operating room or with a portable C-arm is preferred.
Depending on the shape of the aneurysm in the arch, the proximal end of the stentgraft or FET anastomosis may lie anywhere from the distal ascending aorta to beyond the left subclavian artery. The most common position has become the segment between the left common carotid and left subclavian arteries. This provides the advantage of a more manageable suture line with regards to hemostasis, and also avoids excessive manipulation of the left recurrent laryngeal nerve. It is critical that the adventitia of the native vessel, even if aneurysmal, be included in the proximal end of the anastomosis to the stentgraft device.
Patients with atherosclerotic degenerative aneurysms are at higher risk for atheroembolic stroke than those with other etiologies for their aneurysms. Thus, minimizing manipulation of the aorta prior to circulatory arrest is important for limiting that risk.
FET for aortic dissection
Acute
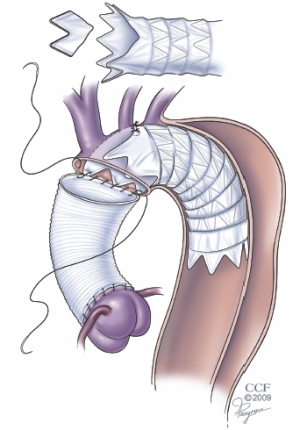
Although several authors have demonstrated that the FET procedure with separate reconstruction of the arch vessels can be performed safely, we have found that the operation can be excessively long for a patient with emergency indications for repair, such as those with an acute dissection. In these situations, we have opted for a simplified FET operation that still involves antegrade delivery of a stentgraft into the descending aorta but leaves the device more proximal in the arch (14). By modifying the commercially available device to accommodate the origins of the arch branch vessels, the FET arch reconstruction can be performed with a single anastomosis (Figure 3). By simplifying the arch reconstruction, we have consistently limited the circulatory arrest time to less than 30 minutes. This technique has proven to be versatile and offers the added advantage of optimizing true lumen perfusion, a characteristic that is particularly important to the patient who presents with malperfusion. This operation is also helpful in the setting of an acute type B dissection where the dissection extends too proximally for a purely endovascular repair. In these cases the ascending aorta, which is vulnerable to late occurrence of dissection or retrograde dissection can also be safely and prophylactically replaced.
Chronic
In patients with chronic dissection, aortic repair is usually performed in an elective setting for aneurysmal degeneration of the false lumen. Most of these patients are survivors of DeBakey type 1 dissections. Others initially presented with DeBakey type 3 dissections extending into the more proximal arch, thereby limiting the seal zone available to a purely endovascular approach. Although an elephant trunk approach can address the proximal disease process well, the distal landing zone in these patients is less predictable because of the presence of distal reentry tears providing persistent retrograde perfusion of the false lumen (Figure 4). For those in whom the proximal descending aneurysm is very large or with many distal re-entry tears, we prefer a two-stage approach with conventional elephant trunk creation followed by endovascular elephant trunk completion (EEC) (10). As an adjunct to the first-stage elephant trunk operation, we include an open fenestration of the distal descending aorta to create a more reliable distal landing zone for the stentgraft device (15).
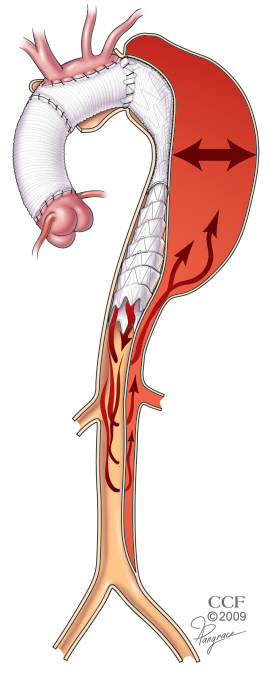
An exception to the two-stage approach in the setting of chronic dissection for patients with minimal distal re-entry tears where the visceral and renal branch arteries arise from the true lumen. For these patients, the surgery is performed just as described above for degenerative aneurysms. The stentgraft device is delivered into the true lumen from above over a previously placed wire that lies within the true lumen. The positioning and course of the wire within the true lumen may be confirmed with the use of intravascular ultrasound (IVUS). This choice is also wise for patients with a very tight angulation at the distal arch that may put the conventional elephant trunk surgical graft at risk for kinking or partial compression.
FET for congenital
Coarctation
As medical therapy and outcomes for primary aortic coarctation have improved, an increasing number of patients are surviving into adulthood. Although the initial repair is durable for decades, a significant number of patients will develop late complications including recurrent coarctation, and development of aneurysms or pseudoaneurysms in the treated segment (16-18). As thoracic imaging has become more widely applied, many patients present with primary coarctation and or associated aneurysm as adolescents or adults. Although conventional repair techniques have been safe for most of these patients, they often have a complex constellation of issues such as hypoplastic arch, dysfunctional bicuspid valve, and ascending aneurysm. For many of these patients, the FET approach has proven to be a versatile option (19). Patients with a hypoplastic arch and a poor proximal landing zone may undergo the FET procedure, which includes patch angioplasty of the arch to accommodate the stentgraft device that is sutured into the augmented proximal landing zone (Figure 5). For others, the FET procedure may be combined with an arch debranching reconstruction, aortic valve replacement, and ascending repair similarly to the procedures done for typical degenerative aneurysms. Using FET in these patients provides the advantage of avoiding a redo thoracotomy. If the patient has a recurrent coarctation, the pre-placed through and through wire provides the ability to perform balloon dilatation of the coarctation before and after device delivery and deployment.

Kommerel’s diverticulum
Another relatively common congenital anomaly that presents with pathology in adulthood is Kommerel’s diverticulum associated with either anomalous right subclavian artery or right-sided arch with anomalous left subclavian artery. The use of an anterior approach through mini-sternotomy with a modified FET repair offers the advantages of avoiding the recurrent laryngeal nerves, release of the ligamentum arteriosum (important for the right sided arch variant), and provides a high-flow proximal site from which the subclavian bypass can be created (20) (Figure 6).
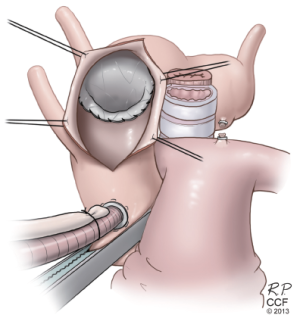
RFET (reverse) for failed TEVAR
Patients are increasingly presenting with proximal aortic complications, including proximal type 1 endoleaks, device migration, and retrograde dissections after undergoing TEVAR. These patients with diffuse aortic pathology can be safely treated with open reconstruction under circulatory arrest by suturing the conventional graft to the previously placed stent graft. Sometimes, this includes the addition of another stent graft device to bring the repair more proximal. We have used the term Reverse Frozen Elephant Trunk (RFET) to describe this modification to the FET technique (21). We have occasionally used this approach in elective settings for patients with particularly complex anatomy such as those with a tight kink in the proximal descending aorta, but more often it is used in urgent situations for complications of prior TEVAR.
Type 1 endoleak
Endoleak from the end of the stentgraft device (type 1) has been one of the most common indications for secondary surgical intervention (22). Most often, this occurs due to poor patient selection at the time of TEVAR who have inadequate proximal landing zones. The FET technique in this situation is typically addressed using SABP and separate debranching of the innominate and left carotid arteries. The left subclavian artery has, more often than not, been covered in these cases in an attempt to extend the proximal landing zone. If the left subclavian artery has not been revascularized previously, then it can be addressed through a sternotomy as an ascending bypass to the supraclavicular subclavian or more distal axillary artery (Figure 7). If it is too difficult to expose, based on assessment of the preoperative CT scan, then the left subclavian artery can be addressed with a left common carotid to subclavian bypass prior to the sternotomy.
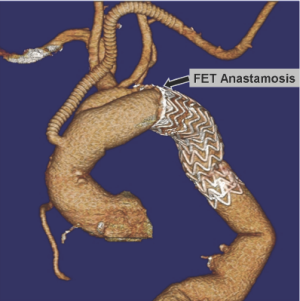
Retrograde dissection
This has become a well-recognized complication of TEVAR, which occurs at a rate of about 2%. While various patient-specific or device-specific risk factors have been suggested, this complication is particularly associated with aortic dissection. This operation can either be done as a simplified RFET with anastomosis of the surgical graft to the stentgraft and native aorta in a hemi-arch fashion, or with separate branching to the supra-aortic vessels (23). If the proximal intimal tear extends across the aortic arch proximal to the end of the stentgraft, the reconstruction may be performed with the use of additional stentgrafts to extend the repair across the tear.
Conclusions
FET repairs have been described in multiple iterations. Like the exchangeable bits available to turn a screw, the various modifications to the FET procedure may be selected to fit the patient with complex arch disease based on the urgency, anatomy, pathology, and indications. As newer devices and systems are specifically developed to perform the FET procedure for various indications, its use will increase. Improvement in outcomes for these complex patients should follow.
Acknowledgements
Disclosure: The author declares no conflict of interest.
References
- Andersen ND, Williams JB, Hanna JM, et al. Results with an algorithmic approach to hybrid repair of the aortic arch. J Vasc Surg 2013;57:655-67; discussion 666-7. [PubMed]
- Gutsche JT, Cheung AT, McGarvey ML, et al. Risk factors for perioperative stroke after thoracic endovascular aortic repair. Ann Thorac Surg 2007;84:1195-200; discussion 1200. [PubMed]
- Kato M, Ohnishi K, Kaneko M, et al. New graft-implanting method for thoracic aortic aneurysm or dissection with a stented graft. Circulation 1996;94:II188-93. [PubMed]
- Karck M, Chavan A, Hagl C, et al. The frozen elephant trunk technique: a new treatment for thoracic aortic aneurysms. J Thorac Cardiovasc Surg 2003;125:1550-3. [PubMed]
- Uchida N, Ishihara H, Shibamura H, et al. Midterm results of extensive primary repair of the thoracic aorta by means of total arch replacement with open stent graft placement for an acute type A aortic dissection. J Thorac Cardiovasc Surg 2006;131:862-7. [PubMed]
- Sun LZ, Qi RD, Chang Q, et al. Surgery for acute type A dissection using total arch replacement combined with stented elephant trunk implantation: experience with 107 patients. J Thorac Cardiovasc Surg 2009;138:1358-62. [PubMed]
- Mestres CA, Tsagakis K, Pacini D, et al. One-stage repair in complex multisegmental thoracic aneurysmal disease: results of a multicentre study. Eur J Cardiothorac Surg 2013. [Epub ahead of print]. [PubMed]
- Shrestha M, Pichlmaier M, Martens A, et al. Total aortic arch replacement with a novel four-branched frozen elephant trunk graft: first-in-man results. Eur J Cardiothorac Surg 2013;43:406-10. [PubMed]
- Greenberg RK, Lu Q, Roselli EE, et al. Contemporary analysis of descending thoracic and thoracoabdominal aneurysm repair: a comparison of endovascular and open techniques. Circulation 2008;118:808-17. [PubMed]
- Roselli EE, Subramanian S, Anderson J, et al. Endovascular versus open elephanttrunk completion for extensive aortic disease. J Thorac Cardiovasc Surg 2013. [Epub ahead of print].;
- Roselli EE, Isabella MA. Frozen elephant trunk procedure. Op Tech in Thorac and CV Surg 2013. [Epub ahead of print].
- Roselli EE, Soltesz EG, Mastracci T, et al. Antegrade delivery of stent grafts to treat complex thoracic aortic disease. Ann Thorac Surg 2010;90:539-46. [PubMed]
- Tsagakis K, Kamler M, Benedik J, et al. Angioscopy--a valuable tool in guiding hybrid stent grafting and decision making during type A aortic dissection surgery. Eur J Cardiothorac Surg 2010;38:507-9. [PubMed]
- Roselli EE, Rafael A, Soltesz EG, et al. Simplified frozen elephant trunk repair for acute DeBakey type I dissection. J Thorac Cardiovasc Surg 2013;145:S197-201. [PubMed]
- Roselli EE, Sepulveda E, Pujara AC, et al. Distal landing zone open fenestration facilitates endovascular elephant trunk completion and false lumen thrombosis. Ann Thorac Surg 2011;92:2078-84. [PubMed]
- Oliver JM, Gallego P, Gonzalez A, et al. Risk factors for aortic complications in adults with coarctation of the aorta. J Am Coll Cardiol 2004;44:1641-7. [PubMed]
- Høimyr H, Christensen TD, Emmertsen K, et al. Surgical repair of coarctation of the aorta: up to 40 years of follow-up. Eur J Cardiothorac Surg 2006;30:910-6. [PubMed]
- Roselli EE, Qureshi A, Idrees J, et al. Open, hybrid, and endovascular treatment for aortic coarctation and postrepair aneurysm in adolescents and adults. Ann Thorac Surg 2012;94:751-6; discussion 757-8. [PubMed]
- Idrees J, Arafat A, Svensson LG, et al. Hybrid repair of aortic aneurysm in patients with previous coarctation. J Thorac Cardiovasc Surg 2013. [Epub ahead of print]. [PubMed]
- Idrees J, Keshavamurthy S, Subramanian S, et al. Hybrid repair of Kommerell diverticulum. J Thorac Cardiovasc Surg 2013. [Epub ahead of print]. [PubMed]
- Lima B, Roselli EE, Soltesz EG, et al. Modified and “reverse” frozen elephant trunk repairs for extensive disease and complications after stent grafting. Ann Thorac Surg 2012;93:103-9; discussion 109. [PubMed]
- Roselli EE, Abdel-Halim M, Johnston DR, et al. Open aortic repair following prior thoracic endovascular aortic repair. Ann Thorac Surg 2013. [Epub ahead of print].
- Idrees J, Arafat A, Svensson L, et al. Repair of retrograde ascending dissection after descending stent grafting. J Thorac Cardiovasc Surg 2013. [Epub ahead of print].