Total arch replacement with frozen elephant trunk technique
Introduction
Our technique for replacing the aortic arch has evolved in recent years from femoral artery cannulation with retrograde cerebral perfusion and deep hypothermic circulatory arrest, to innominate artery cannulation as our first choice, combined with antegrade cerebral perfusion during systemic circulatory arrest with a nasopharyngeal temperature target of 24 °C, and a trifurcated Y-graft (1-3). We have recently reported in high-risk patients that endovascular technology facilitates the repair of arch aneurysms(4). With the help of endovascular stent grafts, we perform total arch replacement with a frozen elephant trunk (FET) in patients whose aneurysm extends through the upper or the entire descending thoracic aorta. If the aneurysm involves the aortic arch and the upper descending thoracic aorta, the repair is performed as a one-stage procedure with antegrade stent delivery of the endograft. If the aneurysm extends into the entire descending aorta, the repair is performed either in one stage with antegrade or retrograde delivery of the endograft or in two stages with retrograde delivery of the stent graft as the second stage of the repair. Our decision to proceed with one-stage versus two-stage repair is based on specific aortic arch anatomy, the complexity of the proximal repair, and the patient’s comorbidities.
Clinical vignette
To illustrate our technique for performing total arch replacement with FET repair, we present a video of this procedure (Video 1). The repair was performed on an 84-year-old patient with 95% occlusion of the right coronary artery (RCA) and a total arch aneurysm extending from the ascending aorta well into the proximal descending thoracic aorta (Figure 1). The patient had a history of pulmonary embolism, prostate cancer, systemic hypertension, moderate pulmonary hypertension, moderate mitral regurgitation, diabetes mellitus, rheumatoid arthritis, and prior endovascular repair of an abdominal aortic aneurysm. The patient’s ejection fraction was depressed, at 30-35%. The patient was symptomatic and was experiencing difficulty swallowing and chest pain. Preoperative evaluation and imaging revealed an ascending aortic aneurysm (approximately 5 cm), an aneurysmal (7 to 8 cm) distal aortic arch, and a proximal descending thoracic aortic aneurysm. Further, coronary angiography revealed hemodynamically significant 95% stenosis of the RCA. Because of the patient’s comorbidities, presentation, preoperative assessment, and imaging findings, we proceeded with the technique described in this report.
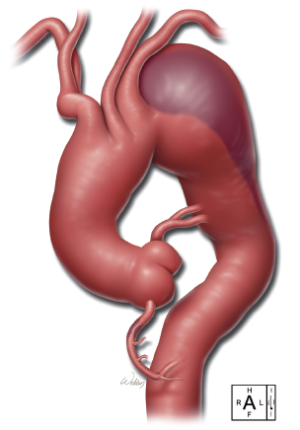
Surgical techniques
A standard median sternotomy was performed with simultaneous saphenous vein endo-harvesting for RCA bypass. Cerebral perfusion pressure was monitored with near-infrared spectroscopy (NIRS) and adjusted accordingly. The anesthesia team placed NIRS probes over the cranium to measure regional cerebral oxygen saturation. All three arch branch vessels were identified. On the basis of the patient’s anatomy, the decision was made to proceed with innominate artery cannulation for arterial inflow. Five thousand units of heparin were administered, and the innominate artery was partially occluded.
An 8 mm Dacron graft was anastomosed end-to-side with 6-0 running Prolene sutures for arterial inflow, reinforced with extra 6-0 Prolene sutures. The anastomosis was performed distally on the innominate artery to avoid interfering with the aortic arch reconstruction. Subsequently, after complete heparinization, a dual-stage venous cannula was placed in the right atrium.
Before cardiopulmonary bypass (CPB) was initiated, left subclavian artery bypass was performed. Because of the size of the arch vessels, and because the trifurcated Y-graft has a limited range of available sizes, a separate 10 mm Dacron graft was used to reconstruct the left subclavian artery. This artery was ligated proximally with a heavy tie and a large clip and divided. The 10 mm Dacron graft was anastomosed end-to-end to the left subclavian artery with running 6-0 Prolene. The anastomosis was reinforced with additional running 5-0 Prolene.
After the left subclavian artery bypass was completed, CPB was initiated. A left ventricular vent was placed via the right superior pulmonary vein and a retrograde cardioplegia cannula into the coronary sinus. Systemic cooling to 24 °C was started, during which period RCA bypass was performed and we continued the reconstruction of the brachiocephalic vessels. A 12×8×8 trifurcated graft was selected, and one of the 8 mm grafts was anastomosed end-to-end to the left common carotid artery. The anastomosis was performed with running 6-0 Prolene and was reinforced with a second continuous layer of 6-0 Prolene. Once the temperature reached 24 °C, the pump flows were turned down to 2 L/min, the innominate artery was occluded with a snare, and circulatory arrest was initiated with unilateral antegrade cerebral perfusion (ACP) via the right common carotid artery. Subsequently, via tubing and a Y-connector to the inflow cannula, a balloon perfusion catheter was connected to the other 8 mm branch of the trifurcated graft to provide direct ACP into the already reconstructed left common carotid artery. The aneurysm was then opened.
We maintained the perfusion pressure at 50 to 60 mmHg during the period of systemic hypothermic circulatory arrest. There were no changes on the NIRS throughout the procedure. After the aneurysm was opened, the 12 mm branch of the trifurcated graft was anastomosed end-to-end to the innominate artery with 5-0 Prolene, and the anastomosis was reinforced with an additional layer of running 6-0 Prolene. After the arch vessels were reconstructed, the balloon perfusion catheter, which was connected to one of the 8 mm branches of the Y-graft, was removed and the flows were maintained from the innominate artery into both the left common carotid artery and the innominate artery.
A 30 mm Dacron skirted elephant trunk (ET) graft was selected to perform the arch replacement, with the ET portion placed in the proximal descending aorta. The anastomosis was performed at the level of the left common carotid artery by using two layers of 2-0 and 3-0 running Prolene, as well as pledgeted 3-0 Prolene mattress sutures. Before the endograft was deployed, the ET graft, which was placed inside the descending thoracic aorta, was stretched with a tri-lobe Gore balloon (W. L. Gore, Flagstaff, AZ). A soft glide wire was advanced under direct vision down the ET in an antegrade fashion and exchanged with a stiff Amplatz wire over a catheter. A 40×20 c-TAG Gore stent graft (W. L. Gore) was advanced antegradely over the stiff wire inside the Dacron ET graft. Before the stent graft was advanced, a slight curve that mimicked the tortuosity of the descending thoracic aorta was applied manually to the stent graft.
The stent graft was deployed with the proximal end of the endograft at the level of the temporary anastomosis of the ET (left common carotid artery). With a bulb syringe, the inside of the stent was irrigated with warm saline to allow further expansion of the nitinol. After deployment, the stent graft was gently expanded further with the Gore tri-lobe balloon under direct vision.
After the FET was completed, the arch graft was cross-clamped, and the 8 mm side branch of the skirted ET was used to perfuse the body distally. The 12 mm graft of the trifurcated graft was sutured end-to-side to the 30 mm ascending portion of the skirted ET graft. Then the cross-clamp was moved proximal to the 12 mm graft of the aorto-innominate bypass, and rewarming was started. We continued preparing the proximal anastomosis at the level of sinotubular junction of the aorta end-to-end with 3-0 and 4-0 running polypropylene in two layers. The saphenous vein graft was attached in the ascending graft with 6-0 polypropylene. Then the cross-clamp was removed, and the pump flows were returned to full flow. During rewarming, a partial occluding clamp was placed on the ascending graft, and the 10 mm Dacron graft to the left subclavian artery was sutured in the ascending graft end-to-side with 4-0 polypropylene (Figure 2).
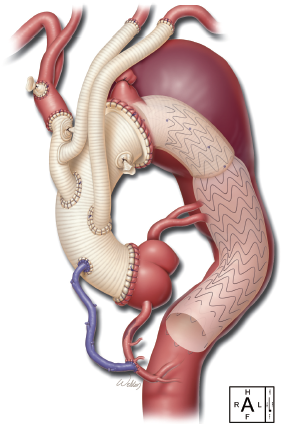
The patient was warmed to 36.5 °C and weaned uneventfully from CPB (Figure 3). Protamine was administered, and surgical hemostasis was performed. The total CPB time (not including circulatory arrest time) was 135 min, and the cerebral isolation time with ACP was 63 min with no cerebral (unprotected) circulatory arrest time. The myocardial ischemia time (the time from the initiation of circulatory arrest until clamp removal, i.e., the period of no direct coronary arterial perfusion) was 102 min.
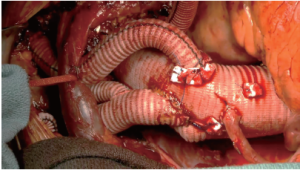
The patient had an uneventful recovery and was neurologically intact when he was extubated the next day. He was discharged on postoperative day 10. Before discharge, transthoracic echocardiography revealed no evidence of aortic valve insufficiency and no pericardial effusion. Computed thoracic angiography showed exclusion of the distal aortic aneurysm, no evidence of endoleak, and complete reconstruction of the arch vessels.
Comments
Extensive thoracic aortic disease is being treated with the traditional ET technique as a two-stage procedure; the second stage is open or endovascular repair, depending on the extent of the thoracoabdominal disease. With FET techniques, the ascending aorta and the arch are replaced in the traditional fashion, and the descending thoracic aorta is repaired with an endograft, which is placed antegradely. These techniques allow a potentially “single-stage” procedure in patients with extensive thoracic disease. Different prostheses have been used in Europe for FET procedures, including the Jotec E-vita and the Vascutek Thoraflex prostheses and other custom-made prostheses (5-7). The reported in-hospital mortality associated with the FET procedure varies from 8.7% to 15%, and the stroke rate ranges from 7.5% to 13%. The incidence of new-onset paraplegia varies from 1% to 21.7% (5-7). In one report of patients who received an FET, a body core temperature of ≥28 °C during circulatory arrest in combination with a prolonged circulatory arrest time of >40 min was an independent predictor of permanent spinal cord injury (6).
The prostheses that are used in Europe for the FET technique are not yet approved for use in the United States. To overcome this obstacle, we use the skirted ET graft, which is used for the traditional ET procedure in combination with the c-TAG Gore stent graft, to perform an FET procedure in patients with disease in the ascending aorta, aortic arch, and proximal descending thoracic aorta. We are very cautious of addressing within the same operation all of the disease in the descending thoracic aorta, given the risk the entire procedure entails and the various postoperative hemodynamic challenges it poses. During the endovascular stage of the procedure, endograft deployment and ballooning are performed under fluoroscopy. The endograft is deployed via a separate 10 mm graft sutured inside the ascending aortic graft for antegrade stent delivery. Retrograde stent delivery is also feasible. The stent is deployed during the rewarming phase and not during systemic hypothermic circulatory arrest, as described here and demonstrated in this video.
Acknowledgements
Joseph C. Brewton III, BS, recorded and edited the video of the procedure. Stephen N. Palmer, PhD, ELS, contributed to the editing of the text.
Disclosure: Dr. Coselli serves as a consultant for Vascutek Ltd., a subsidiary of Terumo Corporation, and receives royalties related to Vascutek. Dr. Coselli serves as Principal Investigator for W. L. Gore & Associates; this pertains to ongoing clinical trials of thoracic stent-grafts. Dr. Preventza has been paid by W. L. Gore & Associates to give lectures about the C-Tag Gore device.
References
- LeMaire SA, Price MD, Parenti JL, et al. Early outcomes after aortic arch replacement by using the Y-graft technique. Ann Thorac Surg 2011;91:700-7. [PubMed]
- Spielvogel D, Halstead JC, Meier M, et al. Aortic arch replacement using a trifurcated graft: simple, versatile, and safe. Ann Thorac Surg 2005;80:90-5. [PubMed]
- Preventza O, Bakaeen FG, Stephens EH, et al. Innominate artery cannulation: an alternative to femoral or axillary cannulation for arterial inflow in proximal aortic surgery. J Thorac Cardiovasc Surg 2013;145:S191-6. [PubMed]
- Preventza O, Bakaeen FG, Cervera RD, et al. Deployment of proximal thoracic endograft in zone 0 of the ascending aorta: treatment options and early outcomes for aortic arch aneurysms in a high-risk population. Eur J Cardiothorac Surg 2013;44:446-53. [PubMed]
- Ius F, Fleissner F, Pichlmaier M, et al. Total aortic arch replacement with the frozen elephant trunk technique: 10-year follow-up single-centre experience. Eur J Cardiothorac Surg 2013. [Epub ahead of print]. [PubMed]
- Leontyev S, Borger MA, Etz CD, et al. Experience with the conventional and frozen elephant trunk techniques: a single-centre study. Eur J Cardiothorac Surg 2013. [Epub ahead of print]. [PubMed]
- Di Bartolomeo R, Di Marco L, Armaro A, et al. Treatment of complex disease of the thoracic aorta: the frozen elephant trunk technique with the E-vita open prosthesis. Eur J Cardiothorac Surg 2009;35:671-5; discussion 675-6. [PubMed]





