Keynote Lecture—Transmitral hypertrophic obstructive cardiomyopathy (HOCM) repair
Introduction
In 1957 RC Brock described the syndrome of functional obstruction of the left ventricle (LV) (1). Even 20 years ago, primary hypertrophic obstructive cardiomyopathy (HOCM) was still considered a uniform but life threatening disease. Today, more than 400 different gene mutations are known to be associated with this disease and several treatment options exist.
In a relevant number of patients, intra-cardiac malformations result in specific outflow obstruction leading to a variety of pathophysiological mechanisms causing mainly dyspnea with eventually development of heart failure. Surgical strategies mainly address the relief of the left ventricular outflow tract (LVOT) obstruction with/without additional mitral valve (MV) intervention.
We herein describe our surgical concept to release LVOT obstruction, which immediately prevents systolic anterior motion (SAM) of the anterior MV leaflet and corrects mitral regurgitation (MR). This technique is particularly useful when operating through a minimal invasive access.
Symptoms and pathophysiology
Symptoms of patients with HOCM are not specific. In the majority of cases, patients experience dyspnea, angina and dizziness, which predominantly occur during exercise due to significantly decreased cardiac output. Several pathomechanisms apply solely or in combination: (I) insufficient myocardial oxygen supply because of the very thick and hypertrophied myocardium; (II) the low end-diastolic volume of the LV due to diastolic relaxation disorder; and (III) moderate to severe MR which increases pulmonary pressure. Additional symptoms also include light-headedness, arrhythmias, presyncope and syncope and even sudden death (2).
The underlying structural lesion is a systolic septal bulge which protrudes into the LVOT and malposition of the anterior papillary muscle (PM). The combination of both factors causes SAM of the AML which itself is further aggravated by hyperdynamic left ventricular contraction drag forces, resulting in a Venturi effect (3).
Surgical techniques
A number of different techniques have been described over the past few decades to address LVOT obstruction. Depending on the extent of LV hypertrophy, either extensive myectomy of nearly the entire LV cavity or a local myectomy of subaortic obstruction is required. It is of note that no specific rules apply regarding the amount of resected myocardium other than prevention of LV perforation and/or ventricular septum defect. The common goal is to eliminate any obstruction as much as possible, thus the surgical technique chosen needs to be closely adapted to the patient’s specific pathology.
The initial surgical treatment of HOCM consisted of a simple myectomy (4), which has also been described by Trimble and Bigelow (5,6).
Surgical principles addressing HOCM and SAM
Several surgical principles exist for surgical treatment of HOCM, which are briefly described below. Morrow and co-workers addressed the LVOT obstruction and SAM by performing a subaortic myectomy (7), an approach which anticipated the involvement of the MV into the pathomechanism of LVOT obstruction. This technique was also promoted by DA Cooley and colleagues, who performed a myectomy and simultaneously replaced the MV (8). Prosthetic MV replacement and concomitant myectomy has thus become the standard operation for HOCM patients for decades. In the nineties, FA Schoendube described a novel technique of myectomy which involved “freeing” of the PMs, often “agglutinated” onto the left ventricular walls and fused to each other (9). The Mayo Clinic approach was to perform left ventricular myectomy through an apical access, specifically in patients with mid ventricular and/or apical hypertrophy (10). Finally, Sano described a technique, which also has been published as a modified Konno, modified Rastan-Konno or modified Rastan II procedure: (I) the infundibulum of the right ventricle is incised in an oblique fashion; (II) the infundibular septum is then incised with or without the guide of a right-angled instrument, which is passed through the aortic valve to mark the septal incision; (III) the latter one is then enlarged below the pulmonary valve; (IV) the subaortic muscle is removed with creation of a ventricular septal defect (VSD); (V) the VSD is closed with interrupted pledgeted sutures and pericardial patch and finally (VI) the right ventricular incision is closed directly or with a second patch (11).
Surgical principles for concomitant treatment of SAM and MV regurgitation
Augmentation of the AML to address MV regurgitation and SAM has been described by MJ Kofflard in 1996 (12). In order to combine LV myectomy through the MV and augmentation of the AML, another operation has been described by A Carpentier in 1988 (13). A retention plasty of the AML has been introduced and successfully performed by the German Heart Center Berlin (14). However, several modification and developments of this combined approach exist. The initial concept of partial resuspension of the AML has been described by JS Rankin in 2008 (15). In this operation, the anterior PM and respective chordae are resected, a ‘traditional’ septal myectomy is performed, and a full annuloplasty ring is placed. The MV was repaired by connecting the left aspect of the leaflets to the posterior PMs, using Gore-Tex artificial chords. Our approach widened this existing concept by complete resuspension of the AML with artificial chordae origination from both PM after performing the left ventricular myectomy which can even be performed through a minimal invasive access (16).
Transmitral HOCM repair
The MV is accessed using right lateral mini-thoracotomy. All native chordae of the AML are completely resected. The AML is then flipped towards the intra-atrial septum leading to exposition of the subaortic muscle. An extensive subaortic myectomy is then performed using knife and scissors after placing a stay suture on the muscle bulge (Figure 1).
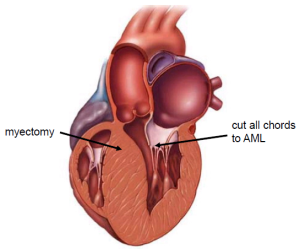
The AML is then completely resuspended from commissure to commissure using the loop technique, which has been described before (17). In brief, pre-manufactured sets of four loops of one length are attached to the relevant PM and secured by teflon pledges (Figure 2). The adequate length of the loops needed is measured by using a custom made caliper. The distal end of this device is placed onto the tip of the postero-medial PM and the respective posterior annulus to calculate the required length of the neo-chords. As a rule, the length of the artificail chordae must be longer than the native chordae in order to increase the mobility of the AML, thus enabling the AML to close at the annular level which leads to prevention of SAM.

Following attachment of the loops to the antero-lateral and postero-medial PM, each individual loop is secured to the corresponding free edge of the AML by an extra 4×0 Gore Tex suture. Each set of loops, therefore originates from ONE PM. A total number of eight loops are finally implanted to resuspend the AML. Care is taken not to cross the midline of the AML with each set of loops (Figure 3).
The MV reconstruction may then be completely with an annuloplasty. In this case we recommend to use a flexible, partial posterior band to allow free opening of the LVOT.
Conclusions
The great variety of different forms of HOCM require an individually tailored approach to each patients specific pathology. In order to increase the surgical armamentarium for HOCM and MV disease treatment, we herein describe our concept of performing LVOT myectomy and complete AML resuspension in these very special patients. When applying this concept, one is able to simultaneously address enlargement of the LVOT, prevent SAM and correct MR with excellent results.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Brock R. Functional obstruction of the left ventricle; acquired aortic subvalvar stenosis. Guys Hosp Rep 1957;106:221-38. [PubMed]
- Fifer MA, Vlahakes GJ. Management of symptoms in hypertrophic cardiomyopathy. Circulation 2008;117:429-39. [PubMed]
- Sherrid MV, Chaudhry FA, Swistel DG. Obstructive hypertrophic cardiomyopathy: echocardiography, pathophysiology, and the continuing evolution of surgery for obstruction. Ann Thorac Surg 2003;75:620-32. [PubMed]
- Goodwin JF, Hollman A, Cleland WP, et al. Obstructive cardiomyopathy simulating aortic stenosis. Br Heart J 1960;22:403-14. [PubMed]
- Trimble AS, Bigelow WG, Wigle ED, et al. Simple and effective surgical approach to muscular subaortic stenosis. Circulation 1964;29 SUPPL:125-9. [PubMed]
- Bigelow WG, Trimble AS, Wigle ED, et al. The treatment of muscular subaortic stenosis. J Thorac Cardiovasc Surg 1974;68:384-92. [PubMed]
- Morrow AG, Reitz BA, Epstein SE, et al. Operative treatment in hypertrophic subaortic stenosis. Techniques, and the results of pre and postoperative assessments in 83 patients. Circulation 1975;52:88-102. [PubMed]
- Cooley DA, Leachman RD, Hallman GL, et al. Idiopathic hypertrophic subaortic stenosis. Surgical treatment including mitral valve replacement. Arch Surg 1971;103:606-9. [PubMed]
- Schoendube FA, Klues HG, Reith S, et al. Long-term clinical and echocardiographic follow-up after surgical correction of hypertrophic obstructive cardiomyopathy with extended myectomy and Reconstruction of the subvalvular mitral apparatus. Circulation 1995;92:II122-7. [PubMed]
- Said SM, Schaff HV, Abel MD, et al. Transapical approach for apical myectomy and relief of midventricular obstruction in hypertrophic cardiomyopathy. J Card Surg 2012;27:443-8. [PubMed]
- Konno S, Imai Y, Iida Y, et al. A new method for prosthetic valve replacement in congenital aortic stenosis associated with hypoplasia of the aortic valve ring. J Thorac Cardiovasc Surg 1975;70:909-17. [PubMed]
- Kofflard MJ, van Herwerden LA, Waldstein DJ, et al. Initial results of combined anterior mitral leaflet extension and myectomy in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol 1996;28:197-202. [PubMed]
- Carpentier A. Le Club Mitrale Newsletter, 1988.
- Delmo Walter EM, Siniawski H, Hetzer R. Sustained improvement after combined anterior mitral valve leaflet retention plasty and septal myectomy in preventing systolic anterior motion in hypertrophic obstructive cardiomyopathy in children. Eur J Cardiothorac Surg 2009;36:546-52. [PubMed]
- Rankin JS, Binford RS, Johnston TS, et al. A new mitral valve repair strategy for hypertrophic obstructive cardiomyopathy. J Heart Valve Dis 2008;17:642-7. [PubMed]
- Seeburger J, Passage J, Borger MA, et al. A new concept for correction of systolic anterior motion and mitral valve regurgitation in patients with hypertrophic obstructive cardiomyopathy. J Thorac Cardiovasc Surg 2010;140:481-3. [PubMed]
- von Oppell UO, Mohr FW. Chordal replacement for both minimally invasive and conventional mitral valve surgery using premeasured Gore-Tex loops. Ann Thorac Surg 2000;70:2166-8. [PubMed]