Concomitant tricuspid valve repair in patients with minimally invasive mitral valve surgery
Introduction
Minimally invasive mitral valve (MIMV) surgery has been routinely used in the Leipzig Heart Center for more than 15 years and has evolved to become a very safe and feasible procedure (1-7). MIMV can be performed in combination with Maze-procedures, atrial septal defect (ASD) closures and tricuspid valve (TV) surgeries. In particular, TV disease has been more frequently discussed in recent times and has therefore become more commonly operated upon in the past few years.
When should the TV be surgically addressed in combination with a mitral valve (MR) procedure? Should TV surgery be performed only in patients with severe TR with a Class I, Level C indication, or should it be routinely performed in patients with mild to moderate secondary TR with a dilated annulus (≥40 mm or ≥21 mm/m2) and a Class IIa, Level C indication? According to the European Society of Cardiology’s (ESC) Guidelines on the Management of Valvular Heart Disease, “adding a tricuspid repair, if indicated during left-sided surgery, does not increase operative risks” (8). Our own experience, comparing the postoperative results of patients with MIMV surgery and moderate TR with and without TV surgery, has confirmed this finding (9).
In this retrospective analysis, we present the peri- and postoperative results in patients undergoing MIMV procedures combined with TV surgery, performed in the Leipzig Heart Center over a 10-year period.
Patients and methods
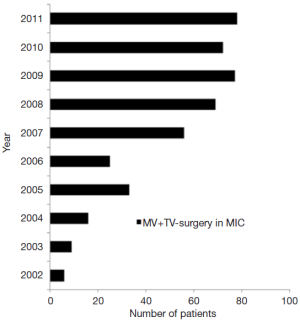
Between January 2002 and December 2011, a total of 441 patients with mitral valve (MV) disease underwent minimally invasive MV with concomitant tricuspid valve (TV) surgery at the Leipzig Heart Center, where there is a consistent increment in the number of procedures every year (Figure 1). Patients with endocarditis were excluded from this study. Indications for MV and TV surgery were based on the recommendations of the ESC on the management of heart valve disease (8).
Pre-, intra- and postoperative data were prospectively entered into a patient data management system and retrospectively analyzed. In addition, a review of patient charts and collection of information from pre-operative and pre-discharge echocardiographic reports was performed and analyzed. MR and TR were quantified by measurement of the vena contracta in the four chamber view (10,11).
The minimally invasive technique for MV and TV surgery via a right anterolateral mini-thoracotomy has been previously described by our group (1,3,4,12). The technique used for MV and TV repair/replacement was at the discretion of the operating surgeon, and was dependent on the preoperative echocardiographic findings.
Follow-up
All patients were contacted by mail and requested to fill out a questionnaire on an annual basis. Patients who did not respond were contacted directly by telephone. If no further information was available, family physicians and/or referring cardiologists were contacted. The mean follow up time was 3.4±2.4 years (range 1 day to 11.0 years).
Statistical evaluation
Results are displayed in the standard format with continuous variables expressed as mean ± standard deviation and categorical data as proportions throughout the manuscript. Risk analysis was done with binary logistic regression analysis. Cumulative survival, as well as the freedom from valve-related reoperation, was calculated by Kaplan-Meier methods. Differences in follow-up were calculated with 95% confidence limits and compared by log rank (Mantel Cox) test.
All statistical analyses were performed using SPSS statistical package 20.0 (IBM SPSS Statistics, Armonk, USA). A P-value <0.05 was considered to be statistically significant.
Results
Demographic data
A total of 441 patients underwent MIMV procedures with concomitant TV surgery. The mean age of all patients was 68.7±10.0 years, the mean logEuroScore was 8.3±7.2% and 184 patients (41.7%) were male (see Table 1). Average NYHA class was 2.7±0.6. Mean left ventricular function was 56.7% and atrial fibrillation was present in two-thirds of the cohort (65.1%). Thirty-two patients (7.3%) had undergone a previous cardiac operation. Annular dilatation of the MV was found in 350 patients (79.4%), 176 patients (39.9%) had prolapse of one or both leaflets and 38 patients (8.6%) had MV stenosis.
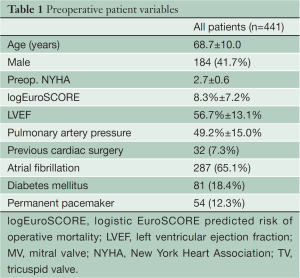
Full table
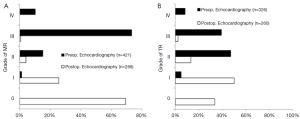
Pure MR was observed in 387 patients (87.8%), of which 328 patients (84.8%) had severe MR on preoperative echocardiography. Preoperative severe TR was found in nearly half of the patients (Figure 2A,B). Operation, cardiopulmonary bypass (CPB) and cross-clamp times were 221.1±50.2, 168.3±40.4 and 97.3±25.3 min, respectively.
MV replacement was necessary in 89 patients (20.2%). MV repair was performed in the remaining patients and was possible in 339 of the 387 patients (96.3%) with pure MR. TV repair using a Kay suture or De Vega-annuloplasty was performed in 13 patients (2.9%), a rigid ring was implanted in 121 patients (27.4%), and a flexible annuloplasty band in 298 patients (67.6%). TV replacement was necessary in the remaining patients. Additional procedures performed were closure of ASDs in 56 patients (12.7%) and Maze-procedures in 272 patients (61.7%).
Perioperative outcomes
Overall 30-day mortality was 4.3% with nineteen deaths. Postoperative re-thoracotomy for bleeding was necessary in 37 patients (8.2%). New postoperative neurological deficit was detected in 10 patients (2.3%), with no deficit lasting permanently. Pre-discharge echocardiography showed no or mild MR in 95.1% and no or mild TR in 84.1% of patients. Detailed preoperative- and predischarge echocardiographic data are depicted in Figure 2A,B.
Survival outcomes
The five-year survival of all patients was 77.2%±2.5% (Figure 3A) and it was significantly higher in patients with preoperative TR ≤2 compared to patients with TR >2 and was independent of the preoperative LVEF or presence of preoperative pulmonary hypertension. However, patients with TR ≤2 were significantly younger than patients with TR >2 (67.2±20.3 vs. 69.8±9.9 years, P=0.02). The difference of survival was only seen in the early postoperative follow-up (Figure 3B).
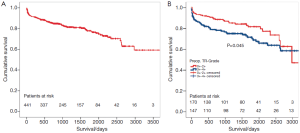
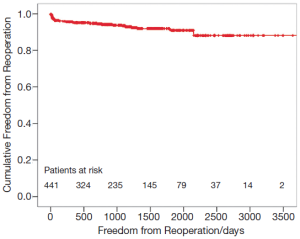
The preoperative MR did not influence the survival of patients. Mid-term freedom from TV-related reoperation is shown in Figure 4. After five years, freedom from TV-related reoperation was 91.0%±1.8% for all patients.
Discussion
Mitral and tricuspid valve surgery through a minimally invasive approach is safe and feasible. Operation, bypass and cross-clamp times are acceptable, and show that the minimally invasive approach is a good alternative to median sternotomy.
The grade of MR did not influence the postoperative survival. In contrast, we found a significant lower survival rate in patients with preoperative TR >2+ as compared to patients with TR ≤2+ (especially in the early postoperative period), which was independent of the preoperative LVEF or presence of preoperative pulmonary hypertension. Patients with a higher grade of TR were significantly older than those with a lower grade, which was similar to the observations of the Framingham study (13). Since the pathology of TV dysfunction is not as well understood as that of the MV, the following discussion is focused on the TV.
In 1967, Braunwald et al. published a paper that influenced the treatment of TV failure for many years. They showed a reduction in TR after MV replacement in patients with MV stenosis at 30 months follow-up (14). In the last few years, many papers have been published on the development of late TR, focusing on its etiology, treatment and prognosis. Late TR has been noted to evolve in some instances up to 20 years after MV/left-sided surgery (15-25). These patients often present very late for reoperations, most often when right ventricular dysfunction has already set in.
According to the Society of Thoracic Surgeons, TV surgery is a valve operation with the highest operative risk (26). Bernal reported a hospital mortality of 35.1% in patients undergoing reoperations on the TV (27). Due to the above-mentioned factors, the benefit of performing concomitant TV surgery with MV surgery became more pronounced in avoiding the development of late TR after left-sided surgery.
The current ESC-guidelines recommend TV surgery in patients with severe TR (class I, Level C) and in patients with mild or moderate secondary TR, with an annulus ≥40 mm or 21 mm/m2 in the echocardiographic four-chamber view at the time of left-sided surgery. It is further stressed that that additional TV surgery does not elevate the operative risk (8). According to the guidelines of the American College of Cardiology (ACC) and American Heart Association (AHA), TV surgery for MV surgical patients who have less than severe TR, pulmonary hypertension or mitral annular dilatation, is a Class IIb, Level C indication (28).
In our institution, concomitant TV repair with left-sided surgery is preferred if the TV annular diameter is ≥40 mm in the four chamber view on echocardiograph. Dreyfus et al. described an increase in the degree of TR by more than two grades during follow-up in 48% of patients undergoing MV surgery without initial concomitant TR (29). Shiran et al. recommended a TV annuloplasty for annular diameters ≥35 mm regardless of TR in patients with rheumatic valve disease, ischemic cardiomyopathy, or functional MR due to dilated cardiomyopathy to avoid development of TR with irreversible right ventricular dysfunction (30). Although this approach seems aggressive, a study by Chopra et al. found a tricuspid annular diameter of 21 mm/m2 to be the best method of differentiating severe from non-severe TR (31). This would correspond to a TV annular diameter of 36mm in an average person in his country (30). If one followed the convention of performing TV repair for a tricuspid annular diameter of 21 mm/m2, a patient with a height of 175 cm and weight of 75 kg would have an TV annuloplasty at a TV annular diameter ≥40 mm, whereas a patient with a height of 160 cm and weight of 60 kg would have a repair at a TV annular diameter ≥35 mm.
In our study, pre-discharge echocardiography revealed residual moderate MR in 4.1% and severe MR in 0.7% of patients. Additionally, we found moderate TR in 13.5% and severe TR in 2.3% of patients. McCarthy reported postoperative severe TR in 14% of patients after TV repair (32).
What are the causes of these failures? In the last few years we have focused on the technique used to repair the TV valve: a De Vega, a rigid ring or a flexible band annuloplasty. Many studies have shown that the ring annuloplasty produces better results than suture annuloplasty (32,33). We use the flexible band for the smooth annulus in our centre because previously we have found a 9-fold higher ring dehiscence in patients undergoing TV repairs with rigid rings than in those with flexible rings. Although ring dehiscence mostly did not produce a hemodynamically relevant TR, we believe that the smooth annulus of the TV should be addressed with a flexible band rather than with a rigid ring (34). Another cause of TR is a pacemaker lead in patients with pacemakers. Should the lead always be replaced, as recommended by McCarthy and colleagues (32)? If so, this would be necessary in 12% of our patients. However, based on the results published by our group, the pacemaker leads are not routinely implanted unless they interfere with the movement of the TV (35).
Ring size is another important consideration in achieving a durable and successful TV repair. Dreyfus suggested the use of a 32 mm ring for women and a 34 mm ring for men (29). Filsoufi and co-workers reported the results of TV repair with the Carpentier-Edwards MC3-ring, in which their group frequently used ring sizes of 26 or 28 mm (36). Another approach reported by Rogers et al. (37) would be to choose the size of the TV ring or band based on the ring size used to repair the MV. This would be better adapted to each individual patient.
In the future, surgeons should focus not only on the operative technique, but also on the preoperative echocardiographic diagnosis and indication for TV surgery. Apart from annulus diameter and severity of TR, Tirone David pointed out that information about the tethering of leaflets, length of the leaflets, area of the annulus and leaflets, RV volume and function should also be obtained (38). Preoperative echocardiography is a very good tool for assessing and evaluating the MV and its dysfunction, and in experienced hands, it is also possible to determine the perfect ring size for the MV. In contrast, the anatomy and function of the TV and geometry of the right ventricle are much more difficult to determine relative to that of the MV and left ventricle. Routinely performed 2D echocardiographic TV and RV measurements are frequently indirect, sometimes incorrect and largely dependent on pre- and afterload conditions. However, the technique of 3D-echocardiography will certainly allow for more precision in the assessment of the TV in the future.
With respect to the indications for TV surgery, it should not be forgotten that concomitant TV repair in patients with less than severe TR is a prophylactic operation and that TR after MV surgery is a long-term problem. Furthermore, not every type of MV dysfunction leads to late TR—patients with rheumatic and ischemic MV dysfunction and MR due to dilated cardiomyopathy tend to develop late TR. Conversely, patients with MR due leaflet prolapse or myxomatous disease do not seem to be predisposed to late TR (19,29,38,39). Therefore surgeons must decide which patients would benefit most from prophylactic TV surgery.
The main limitation of the current study is its retrospective, non-randomized nature. However, the current study represents one of the largest series of patients undergoing MIMV surgery with concomitant TV surgery to date and therefore may add further insights to this important issue. Another important limitation is the lack of detailed preoperative and follow-up echocardiographic data. This limitation is partially due to the fact that measurements of the tricuspid annulus, and tricuspid annular plane systolic excursion (TAPSE) were not routinely performed and recorded in the earlier years of the study. In addition, there is very little data on TV leaflet prolapse or tethering available. Moreover, nearly one-third of our MIMV patients are referred from other regions of the country, which makes it logistically impossible for us to perform the optimal echocardiographic follow-up at our institution.
In conclusion, MIMV surgery combined with TV repair can be performed safely and effectively and is a good alternative to median sternotomy. Surgeons should concentrate more on a detailed preoperative echocardiographic diagnosis of TV dysfunction, determine the indication for surgery and choose an appropriate operative technique to attain the best possible outcomes after TV repair—at least as close as possible to those of MV surgery.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Mohr FW, Falk V, Diegeler A, et al. Minimally invasive port-access mitral valve surgery. J Thorac Cardiovasc Surg 1998;115:567-74; discussion 574-6. [PubMed]
- Falk V, Seeburger J, Czesla M, et al. How does the use of polytetrafluoroethylene neochordae for posterior mitral valve prolapse (loop technique) compare with leaflet resection? A prospective randomized trial. J Thorac Cardiovasc Surg 2008;136:1205; discussion 1205-6. [PubMed]
- Kuntze T, Borger MA, Falk V, et al. Early and mid-term results of mitral valve repair using premeasured Gore-Tex loops (‘loop technique’). Eur J Cardiothorac Surg 2008;33:566-72. [PubMed]
- Seeburger J, Borger MA, Falk V, et al. Minimal invasive mitral valve repair for mitral regurgitation: results of 1339 consecutive patients. Eur J Cardiothorac Surg 2008;34:760-5. [PubMed]
- Seeburger J, Kuntze T, Mohr FW. Gore-tex chordoplasty in degenerative mitral valve repair. Semin Thorac Cardiovasc Surg 2007;19:111-5. [PubMed]
- Vollroth M, Seeburger J, Garbade J, et al. Minimally invasive mitral valve surgery is a very safe procedure with very low rates of conversion to full sternotomy. Eur J Cardiothorac Surg 2012;42:e13-5; discusson e16.
- Pfannmüller B, Seeburger J, Misfeld M, et al. Minimally invasive mitral valve repair for anterior leaflet prolapse. J Thorac Cardiovasc Surg 2013;146:109-13. [PubMed]
- Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC), European Association for Cardio-Thoracic Surgery (EACTS), Vahanian A, et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 2012;33:2451-96. [PubMed]
- Pfannmueller B, Verevkin A, Borger MA, et al. Role of tricuspid valve repair for moderate tricuspid regurgitation during minimally invasive mitral valve surgery. Thorac Cardiovasc Surg 2013;61:386-91. [PubMed]
- Fehske W, Omran H, Manz M, et al. Color-coded Doppler imaging of the vena contracta as a basis for quantification of pure mitral regurgitation. Am J Cardiol 1994;73:268-74. [PubMed]
- Tribouilloy CM, Enriquez-Sarano M, Bailey KR, et al. Quantification of tricuspid regurgitation by measuring the width of the vena contracta with Doppler color flow imaging: a clinical study. J Am Coll Cardiol 2000;36:472-8. [PubMed]
- Seeburger J, Borger MA, Passage J, et al. Minimally invasive isolated tricuspid valve surgery. J Heart Valve Dis 2010;19:189-92; discussion 193. [PubMed]
- Singh JP, Evans JC, Levy D, et al. Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study). Am J Cardiol 1999;83:897-902. [PubMed]
- Braunwald NS, Ross J Jr, Morrow AG. Conservative management of tricuspid regurgitation in patients undergoing mitral valve replacement. Circulation 1967;35:I63-9. [PubMed]
- Kwak JJ, Kim YJ, Kim MK, et al. Development of tricuspid regurgitation late after left-sided valve surgery: a single-center experience with long-term echocardiographic examinations. Am Heart J 2008;155:732-7. [PubMed]
- Kwon DA, Park JS, Chang HJ, et al. Prediction of outcome in patients undergoing surgery for severe tricuspid regurgitation following mitral valve surgery and role of tricuspid annular systolic velocity. Am J Cardiol 2006;98:659-61. [PubMed]
- Boyaci A, Gokce V, Topaloglu S, et al. Outcome of significant functional tricuspid regurgitation late after mitral valve replacement for predominant rheumatic mitral stenosis. Angiology 2007;58:336-42. [PubMed]
- Izumi C, Miyake M, Takahashi S, et al. Progression of isolated tricuspid regurgitation late after left-sided valve surgery. Clinical features and mechanisms. Circ J 2011;75:2902-7. [PubMed]
- De Bonis M, Lapenna E, Sorrentino F, et al. Evolution of tricuspid regurgitation after mitral valve repair for functional mitral regurgitation in dilated cardiomyopathy. Eur J Cardiothorac Surg 2008;33:600-6. [PubMed]
- García Fuster R, Vázquez A, Peláez AG, et al. Factors for development of late significant tricuspid regurgitation after mitral valve replacement: the impact of subvalvular preservation. Eur J Cardiothorac Surg 2011;39:866-74; discussion 874. [PubMed]
- Gursoy M, Hatemi AC. Mild-to-moderate functional tricuspid regurgitation in patients undergoing valve replacement for rheumatic mitral disease: the influence of tricuspid valve repair on clinical and echocardiographic outcomes. Heart 2012;98:1181-author reply 1181-2. [PubMed]
- Song H, Kim MJ, Chung CH, et al. Factors associated with development of late significant tricuspid regurgitation after successful left-sided valve surgery. Heart 2009;95:931-6. [PubMed]
- Shi KH, Xuan HY, Zhang F, et al. Evolution of tricuspid regurgitation after mitral valve surgery for patients with moderate-or-less functional tricuspid regurgitation. Heart Surg Forum 2012;15:E121-6. [PubMed]
- Katsi V, Raftopoulos L, Aggeli C, et al. Tricuspid regurgitation after successful mitral valve surgery. Interact Cardiovasc Thorac Surg 2012;15:102-8. [PubMed]
- Johnston SR, Freeman WK, Schaff HV, et al. Severe tricuspid regurgitation after mitral valve repair: diagnosis by intraoperative transesophageal echocardiography. J Am Soc Echocardiogr 1990;3:416-9. [PubMed]
- Rankin JS, Hammill BG, Ferguson TB Jr, et al. Determinants of operative mortality in valvular heart surgery. J Thorac Cardiovasc Surg 2006;131:547-57. [PubMed]
- Bernal JM, Morales D, Revuelta C, et al. Reoperations after tricuspid valve repair. J Thorac Cardiovasc Surg 2005;130:498-503. [PubMed]
- Bonow RO, Carabello BA, Chatterjee K, et al. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2008;52:e1-142. [PubMed]
- Dreyfus GD, Corbi PJ, Chan KM, et al. Secondary tricuspid regurgitation or dilatation: which should be the criteria for surgical repair? Ann Thorac Surg 2005;79:127-32. [PubMed]
- Shiran A, Sagie A. Tricuspid regurgitation in mitral valve disease incidence, prognostic implications, mechanism, and management. J Am Coll Cardiol 2009;53:401-8. [PubMed]
- Chopra HK, Nanda NC, Fan P, et al. Can two-dimensional echocardiography and Doppler color flow mapping identify the need for tricuspid valve repair? J Am Coll Cardiol 1989;14:1266-74. [PubMed]
- McCarthy PM, Bhudia SK, Rajeswaran J, et al. Tricuspid valve repair: durability and risk factors for failure. J Thorac Cardiovasc Surg 2004;127:674-85. [PubMed]
- Tang GH, David TE, Singh SK, et al. Tricuspid valve repair with an annuloplasty ring results in improved long-term outcomes. Circulation 2006;114:I577-81. [PubMed]
- Pfannmüller B, Doenst T, Eberhardt K, et al. Increased risk of dehiscence after tricuspid valve repair with rigid annuloplasty rings. J Thorac Cardiovasc Surg 2012;143:1050-5. [PubMed]
- Pfannmueller B, Hirnle G, Seeburger J, et al. Tricuspid valve repair in the presence of a permanent ventricular pacemaker lead. Eur J Cardiothorac Surg 2011;39:657-61. [PubMed]
- Filsoufi F, Salzberg SP, Coutu M, et al. A three-dimensional ring annuloplasty for the treatment of tricuspid regurgitation. Ann Thorac Surg 2006;81:2273-7. [PubMed]
- Rogers JH, Bolling SF. Valve repair for functional tricuspid valve regurgitation: anatomical and surgical considerations. Semin Thorac Cardiovasc Surg 2010;22:84-9. [PubMed]
- David TE. Functional tricuspid regurgitation: a perplexing problem. J Am Soc Echocardiogr 2009;22:904-6. [PubMed]
- Matsunaga A, Duran CM. Progression of tricuspid regurgitation after repaired functional ischemic mitral regurgitation. Circulation 2005;112:I453-7. [PubMed]