Barlow’s mitral valve disease: results of conventional and minimally invasive repair approaches
Introduction
Fibroelastic deficiency and Barlow’s disease are the two most common etiologies of degenerative mitral valve (MV) disease, often leading to significant mitral regurgitation (MR). Barlow’s disease is characterized by pronounced annular dilatation, bileaflet prolapse and/or billowing, hooding, and the presence of thick, spongy leaflets due to excessive myxomatous tissue proliferation with or without calcification (1). Barlow’s pathology constitutes a challenge for surgeons performing MV repair. Achieving a durable surgical result may be a formidable task in these frequently young and otherwise healthy patients.
Minimally invasive (MIS) MV surgery has proven to be feasible and technically acceptable for a wide range of pathologies. Mounting data in the literature supports the hypothesis that MIS can provide at least equivalent results for surgical correction of MR with several associated clinical benefits (2-4). Although MIS should be predominantly reserved for non-complex pathologies (e.g., FED, isolated P2 prolapse) during the initial part of the surgeon’s learning curve (5), it has also been demonstrated that such techniques can be safely and effectively utilized for complex mitral pathologies (e.g., bileaflet prolapse and Barlow’s disease) in high volume centers (3,4,6-10). The objective of this paper was to review the available results of MV repair in Barlow’s disease and/or bileaflet MV prolapse via conventional versus minimally-invasive approaches.
Established repair techniques in Barlow’s disease
First described by John B. Barlow (11,12), Barlow’s disease is characterized by excessive myxomatous tissue—the hallmark of this pathology—as well as annular dilatation, leaflet thickening, bileaflet prolapse, chordal lengthening and, not infrequently, valvular tissue calcification. Additionally, as pointed out by Hutchins (13), Barlow’s valve is frequently associated with disjunction of the mitral annulus fibrosus. The resultant atrial displacement of the mitral leaflet attachment may lead to leaflet hypermobility and subsequent excessive mucoid degeneration. Histologically, Barlow’s valve is characterized by myxoid infiltration, which destroys the 3-layer leaflet architecture, and collagen alterations (14).
MV repair in Barlow’s disease is particularly challenging because of extensive bileaflet billowing, which makes it difficult for the surgeon to find a normal “reference point” and thereby complicates valve analysis and repair planning. Nevertheless, there is a variety of well described surgical techniques for successful repair of this challenging pathology (15). The one consistent element of various Barlow’s repair techniques is the use of a large annuloplasty ring. As suggested by Adams et al. (16), the definition of a true Barlow’s valve should be limited to those requiring a complete ring of size 36 mm or larger. Beyond this one consistent element, a wide variety of leaflet repair techniques have been successfully implemented including leaflet resection, neochord formation, plication and other techniques.
Most of the resectional techniques described for Barlow’s and bileaflet MV repair are well established and carry very good long-term results (17). One of the most traditional and well known of such techniques incorporates a complete resection of the middle scallop of the posterior mitral leaflet (PML) followed by a sliding or a folding plasty with the remaining lateral scallops. It might be supplemented furthermore with either a triangular resection of the anterior leaflet (AML) (especially in the cases with a long, localized AML) or a correction of the AML prolapse with polytetrafluoroethylene (PTFE) chordae or loops (18,19). Secondary MV lesions, such as leaflet clefts and minor commissural prolapses, become apparent upon the water-sealing test. In such instances, the clefts are directly closed with Prolene or Cardionyl 5-0 sutures and the residual commissural prolapse can be corrected either by insertion of more artificial chords or by insertion of a vertical mattress stitch (also known as a “magic stitch”). When encountered, calcifications of the annulus should be removed as proposed by Carpentier (20). Although such resectional techniques are well established, they can be technically challenging to perform through a MIS approach.
Perrier’s group first coined the term “respect rather than resect” to describe an alternative to traditional resection techniques (21). The goal of this approach is to correct MV prolapse without excision of leaflet tissue. This can be achieved for the PML with the use of PTFE chordae or Loops, with adjustment of their length so that the PML remains nearly vertical, posterior and parallel to the posterior wall of the left ventricle in the inflow region. This transforms the PML into a smooth, regular and vertical buttress against which the AML will come into apposition. The use of PTFE neochordae has also been described for correction of AML prolapse, with the Loop technique being particularly valuable for MIS surgery (3-10). The Loops to the AML are approximately 10 mm longer than those applied to the PML because of the increased AML mobility that is required to achieve MV competence. One can envision the AML acting like a “door” and the PML as a “doorframe” for the “respect” methods.
The double-orifice technique described by Alfieri’s group (22) deserves special mention, as this technique drastically simplifies the repair of the Barlow’s valve. In this approach, the edge-to-edge approximation of the middle scallop of the anterior and posterior leaflet allows the elimination of most of the mitral insufficiency, while the residual smaller regurgitant jets are effectively corrected by the association of a ring annuloplasty. One may argue that the Alfieri technique is counterintuitive for a pathology characterized by elongation of both the subvalvular apparatus and the leaflet height. Nevertheless, a properly placed edge-to-edge stitch that encompasses a large amount of leaflet tissue, in combination with a very large complete ring, can lead to a marked shortening of the height of both leaflets and a lowering of the coaptation point to within the left ventricle. Because of its technical simplicity and reproducibility, this technique can be easily applied through a MIS approach (23).
Repair outcomes in conventional approaches
In spite of challenges presented by MV repair in Barlow’s disease, referral of these cases to selected centers has helped MV surgeons around the world to obtain excellent short- and long-term results for this difficult pathology. Table 1 summarizes the outcomes of the largest series for MV repair in Barlow’s disease or bileaflet prolapse via conventional sternotomy or MIS approaches. David and associates (24) reviewed their results in 701 patients with mitral prolapse, of which 250 patients were operated on for bileaflet prolapse including Barlow’s disease. They report freedom from moderate or severe MR rates at 12 years of 80%±4% for posterior, 65%±8% for anterior, and 67%±6% for bileaflet prolapse.
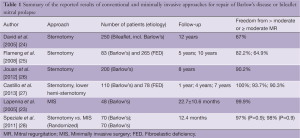
Full table
Another series by Flameng et al. (25) describes a series of 348 patients who have undergone MV repair for degenerative MR. In this series comprising patients with Barlow’s disease (n=83) and fibroelastic deficiency (n=265), freedom from recurrent MR (>2+) was 82.2% at five years and 64.9% at ten years for the entire group. However, recurrent MR was much more common in patients with Barlow’s disease with a linearized recurrence rate of 6.0% per year (compared to 2.3% for fibroelastic deficiency).
Jouan et al. (26) described 200 patients with Barlow’s disease who underwent MV repair via a conventional sternotomy. A successful repair was achieved in 94.7% (179/189) of non-redo patients. Operative mortality was 1.5% (n=3) and the overall survival at eight years was 88.6%±3.1%. The freedom from MV reoperation at eight years was 95.3%±1.7% and the freedom from late recurrent MR (>2+) was 90.2%±3.1%.
Castillo, Adams et al. (27) described their results in 188 consecutive patients who underwent surgery for degenerative anterior or bileaflet mitral leaflet prolapse (Barlow’s disease in 110 patients and fibroelastic deficiency in 78 patients). Freedom from more than moderate MR was 100% at one year, 93.7%±2.2% at four years, and 90.3%±3.7% at seven years. All procedures were performed through either a median sternotomy or a lower hemisternotomy approach.
Repair outcomes in minimally invasive approaches
MIS MV surgery has slowly gained increasing acceptance within the cardiac surgical community and is experiencing a growing demand among patients and referring physicians. In Germany, over 40% of patients currently undergoing MV surgery are operated on with a MIS approach (29). MIS facilitates earlier resumption of normal activities, which is associated with improved cosmesis, and may reduce postoperative pain, blood loss, and hospital length of stay (4,7-10,30,31).
A MIS approach is defined as an operation utilizing a chest wall incision other than a median sternotomy (32). Most commonly employed approaches include right mini-thoracotomy, right thoracic incisions for the robotically-assisted surgery and partial sternotomy. At the vast majority of centers employing a MIS approach to MV repair, including our own, the standard approach is via a small (5-7 cm in length) anterolateral mini-thoracotomy as described in detail elsewhere (2,18). Briefly, cardiopulmonary bypass (CPB) is instituted via femoral arterial and venous cannulation. The aorta is cross-clamped with a Chitwood clamp and myocardial protection is achieved with mild hypothermia (34 °C) and antegrade delivery of cardioplegia. The left atrium is then opened posterior to the interatrial groove and a left atrial retractor is used to expose the MV. The stepwise approach to the Barlow’s valve repair is similar to that used during conventional sternotomy (15). Essentially, the entire spectrum of repair techniques can be utilized via a MIS approach including ring annuloplasty, leaflet resection with or without sliding annuloplasty, the “Loop” technique with premeasured Gore-Tex neo-chordae, chordal transfer, commissural plication, the edge-to-edge (“Alfieri”) technique, cleft closure and calcium debridement. The edge-to-edge technique and the use of artificial chords (including the “Loop” technique) to correct leaflet prolapse are simpler and less technically challenging to perform, and therefore are more frequently utilized in MIS Barlow’s operations. Several studies have reported on the results of MIS approaches to Barlow’s MV repair. Lapenna et al. (23) described 48 such patients, all of whom underwent an “edge-to-edge” repair via a small right anterolateral thoracotomy and peripheral femoral vessel cannulation for CPB. MV repair was successful in 100% of patients. At a mean follow-up time of 22.7±10.6 months, no residual MR was detected on echocardiography in 33 (68.7%) patients and mild insufficiency was found in 15 (31.2%). At the time of follow-up, survival rate and freedom from reoperation was 100% and all patients were in NYHA class I.
Speziale et al. (28) randomized patients with Barlow’s disease to undergo conventional open repair via median sternotomy or MIS repair. In both groups (each group comprised of 70 patients), MV repair was performed using Gore-Tex neo-chordae for chordal reimplantation on both leaflets. MV repair was successful in 98.5% of MIS patients and 100% of median sternotomy patients. Although operative and CPB times were longer in MIS patients, no differences in safety or efficacy outcomes were observed between the two groups, including freedom from reoperation (100% in each group) and freedom from moderate or worse MR (98% vs. 97%) at 1-year follow-up .
We have also recently examined our outcomes for MIS surgery in patients with Barlow’s disease (33). A total of 145 Barlow’s patients underwent MIS MV surgery at our institution over an 11-year period. MV repair was successfully performed in 95% of patients using Loop neochordae in 72%, PML resection in 30%, Alfieri stitch in 18%, commissural plication in 9%, chordal transfer in 9% and AML resection in 7% of patients. The perioperative rates of morbidity and mortality were very low. Long-term follow-up revealed a ten-year survival rate of 88%, while ten-year freedom from MV reoperation or recurrent (>2+) MR amongst survivors was 90% and 88%, respectively. Such results confirm that Barlow’s pathology can be successfully treated with MIS surgery using a variety of surgical techniques.
Conclusions
Growing evidence demonstrates that the results of the MIS approach for MV surgery are at least as good as those achieved with conventional surgery, with several clinical advantages over a full sternotomy approach. One critique of MIS surgery has been its suitability to treat complex MV pathology, such as Barlow’s disease. We herein describe very good early and long-term results for the MIS approach in Barlow’s patients operated on in select, large volume centers. The fact that these case series come from centers with a large MIS MV repair experience should not be overlooked, since we and others have clearly shown a learning curve effect associated with MIS surgery (5). Complex MV pathologies such as Barlow’s valve should not be attempted during the early portion of the MIS learning curve.
Patients with severe MR due to Barlow’s disease are often asymptomatic and may be reluctant to undergo a median sternotomy. The small right anterolateral incision is not only cosmetically appealing, but also allows patients to return faster to an active life. A multitude of MV repair techniques can be employed via the MIS approach, although the edge-to-edge and neochordae/Loop technique are technically easier through a small incision.
Prêtre (34) once noted that “one should never lose sight of our cardinal priority: the cosmetic must be achieved primarily on the heart, not on the skin”. Undoubtedly, the success rate and durability of the MV repair should be the first priority in patients with MR. However, the evolution of technology, the increasingly widespread adoption of MIS valve surgery, the dissipation of knowledge within the cardiac surgery community, and the increase in the cardiac surgeon’s armamentarium of MV repair techniques have all made it possible to achieve excellent results through a MIS approach, even in patients with complex mitral pathology. We must also acknowledge the fact that the rapid advancements in transcutaneous valve techniques are likely to result in an even larger proportion of patients requesting MIS surgery in the foreseeable future.
Summary
MIS MV repair is feasible in patients with Barlow’s disease. Given the previously demonstrated clinical benefits of MIS surgery, this approach may be strongly considered for patients presenting with Barlow’s disease. However, the MIS approach is associated with a definite learning curve and, therefore, patients with complex MV pathology, including Barlow’s, should be referred to centers with established experience in complex MIS MV repair.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Anyanwu AC, Adams DH. Etiologic classification of degenerative mitral valve disease: Barlow’s disease and fibroelastic deficiency. Semin Thorac Cardiovasc Surg 2007;19:90-6. [PubMed]
- Mohr FW, Onnasch JF, Falk V, et al. The evolution of minimally invasive valve surgery--2 year experience. Eur J Cardiothorac Surg 1999;15:233-8; discussion238-9.
- Seeburger J, Borger MA, Falk V, et al. Minimal invasive mitral valve repair for mitral regurgitation: results of 1339 consecutive patients. Eur J Cardiothorac Surg 2008;34:760-5. [PubMed]
- Goldstone AB, Atluri P, Szeto WY, et al. Minimally invasive approach provides at least equivalent results for surgical correction of mitral regurgitation: a propensity-matched comparison. J Thorac Cardiovasc Surg 2013;145:748-56. [PubMed]
- Holzhey DM, Seeburger J, Misfeld M, et al. Learning minimally invasive mitral valve surgery: a cumulative sum sequential probability analysis of 3895 operations from a single high-volume center. Circulation 2013;128:483-91. [PubMed]
- Kuntze T, Borger MA, Falk V, et al. Early and mid-term results of mitral valve repair using premeasured Gore-Tex loops (‘loop technique’). Eur J Cardiothorac Surg 2008;33:566-72. [PubMed]
- Suri RM, Burkhart HM, Rehfeldt KH, et al. Robotic mitral valve repair for all categories of leaflet prolapse: improving patient appeal and advancing standard of care. Mayo Clin Proc 2011;86:838-44. [PubMed]
- McClure RS, Cohn LH, Wiegerinck E, et al. Early and late outcomes in minimally invasive mitral valve repair: an eleven-year experience in 707 patients. J Thorac Cardiovasc Surg 2009;137:70-5. [PubMed]
- Rodriguez E, Nifong LW, Chu MW, et al. Robotic mitral valve repair for anterior leaflet and bileaflet prolapse. Ann Thorac Surg 2008;85:438-44; discussion 444. [PubMed]
- Casselman FP, Van Slycke S, Dom H, et al. Endoscopic mitral valve repair: feasible, reproducible, and durable. J Thorac Cardiovasc Surg 2003;125:273-82. [PubMed]
- Barlow JB, Bosman CK. Aneurysmal protrusion of the posterior leaflet of the mitral valve. An auscultatory-electrocardiographic syndrome. Am Heart J 1966;71:166-78. [PubMed]
- Barlow JB, Bosman CK, Pocock WA, et al. Late systolic murmurs and non-ejection (“mid-late”) systolic clicks. An analysis of 90 patients. Br Heart J 1968;30:203-18. [PubMed]
- Hutchins GM, Moore GW, Skoog DK. The association of floppy mitral valve with disjunction of the mitral annulus fibrosus. N Engl J Med 1986;314:535-40. [PubMed]
- Fornes P, Heudes D, Fuzellier JF, et al. Correlation between clinical and histologic patterns of degenerative mitral valve insufficiency: a histomorphometric study of 130 excised segments. Cardiovasc Pathol 1999;8:81-92. [PubMed]
- Borger MA, Mohr FW. Repair of bileaflet prolapse in Barlow syndrome. Semin Thorac Cardiovasc Surg 2010;22:174-8. [PubMed]
- Adams DH, Anyanwu AC, Rahmanian PB, et al. Large annuloplasty rings facilitate mitral valve repair in Barlow’s disease. Ann Thorac Surg 2006;82:2096-100; discussion 2101. [PubMed]
- Braunberger E, Deloche A, Berrebi A, et al. Very long-term results (more than 20 years) of valve repair with carpentier’s techniques in nonrheumatic mitral valve insufficiency. Circulation 2001;104:I8-11. [PubMed]
- von Oppell UO, Mohr FW. Chordal replacement for both minimally invasive and conventional mitral valve surgery using premeasured Gore-Tex loops. Ann Thorac Surg 2000;70:2166-8. [PubMed]
- Falk V, Seeburger J, Czesla M, et al. How does the use of polytetrafluoroethylene neochordae for posterior mitral valve prolapse (loop technique) compare with leaflet resection? A prospective randomized trial. J Thorac Cardiovasc Surg 2008;136:1205-discussion 1205-6. [PubMed]
- Carpentier AF, Pellerin M, Fuzellier JF, et al. Extensive calcification of the mitral valve anulus: pathology and surgical management. J Thorac Cardiovasc Surg 1996;111:718-29; discussion 729-30. [PubMed]
- Perier P, Hohenberger W, Lakew F, et al. Toward a new paradigm for the Reconstruction of posterior leaflet prolapse: midterm results of the “respect rather than resect” approach. Ann Thorac Surg 2008;86:718-25; discussion 718-25. [PubMed]
- Maisano F, Schreuder JJ, Oppizzi M, et al. The double-orifice technique as a standardized approach to treat mitral regurgitation due to severe myxomatous disease: surgical technique. Eur J Cardiothorac Surg 2000;17:201-5. [PubMed]
- Lapenna E, Torracca L, De Bonis M, et al. Minimally invasive mitral valve repair in the context of Barlow’s disease. Ann Thorac Surg 2005;79:1496-9. [PubMed]
- David TE, Ivanov J, Armstrong S, et al. A comparison of outcomes of mitral valve repair for degenerative disease with posterior, anterior, and bileaflet prolapse. J Thorac Cardiovasc Surg 2005;130:1242-9. [PubMed]
- Flameng W, Meuris B, Herijgers P, et al. Durability of mitral valve repair in Barlow disease versus fibroelastic deficiency. J Thorac Cardiovasc Surg 2008;135:274-82. [PubMed]
- Jouan J, Berrebi A, Chauvaud S, et al. Mitral valve reconstruction in Barlow disease: long-term echographic results and implications for surgical management. J Thorac Cardiovasc Surg 2012;143:S17-20. [PubMed]
- Castillo JG, Anyanwu AC, El-Eshmawi A, et al. All anterior and bileaflet mitral valve prolapses are repairable in the modern era of reconstructive surgery. Eur J Cardiothorac Surg 2013. [Epub ahead of print]. [PubMed]
- Speziale G, Nasso G, Esposito G, et al. Results of mitral valve repair for Barlow disease (bileaflet prolapse) via right minithoracotomy versus conventional median sternotomy: a randomized trial. J Thorac Cardiovasc Surg 2011;142:77-83. [PubMed]
- Funkat AK, Beckmann A, Lewandowski J, et al. Cardiac surgery in Germany during 2011: a report on behalf of the German Society for Thoracic and Cardiovascular Surgery. Thorac Cardiovasc Surg 2012;60:371-82. [PubMed]
- Mihaljevic T, Pattakos G, Gillinov AM, et al. Robotic posterior mitral leaflet repair: neochordal versus resectional techniques. Ann Thorac Surg 2013;95:787-94. [PubMed]
- Iribarne A, Easterwood R, Russo MJ, et al. Comparative effectiveness of minimally invasive versus traditional sternotomy mitral valve surgery in elderly patients. J Thorac Cardiovasc Surg 2012;143:S86-90. [PubMed]
- Rosengart TK, Feldman T, Borger MA, et al. Percutaneous and minimally invasive valve procedures: a scientific statement from the American Heart Association Council on Cardiovascular Surgery and Anesthesia, Council on Clinical Cardiology, Functional Genomics and Translational Biology Interdisciplinary Working Group, and Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation 2008;117:1750-67. [PubMed]
- Borger MA, Kaeding AF, Seeburger J, et al. Minimally invasive mitral valve repair in barlow’s disease: early and long-term results. J Thorac Cardiovasc Surg 2013;
- Prêtre R. Minimal invasive surgery in congenital heart defects: keeping sight of our priority. Eur J Cardiothorac Surg 2012;42:980. [PubMed]





