Value of transesophageal echocardiography (TEE) guidance in minimally invasive mitral valve surgery
At the Albert Einstein College of Medicine in New York in 1979, Matsumoto et al. described the first use of transesophageal echocardiography (TEE) in a 65-year-old woman undergoing open mitral valvuloplasty (1). This marked the start of rapid growth in this emerging technology in cardiac surgery and anesthesiology. Nowadays, perioperative TEE should be used in all patients undergoing surgical mitral valve repair (2,3). The TEE should be incorporated into a comprehensive examination in both the pre- and post-operative periods (4). The detection of new, unexpected findings due to the comprehensive intraoperative TEE examination varies from 4% to 25% and has a huge impact on surgical decision-making (5-8).
In the pre-operative period, TEE should evaluate: (I) the mitral valve apparatus; (II) the function of the mitral valve leaflets and pathological segments; (III) the severity of mitral regurgitation under general anesthesia; (IV) potential risk factors for the surgical repair; and (V) correct placement of the cannulas used for cardiopulmonary bypass (CPB).
The post-operative TEE examination can be divided into the weaning period from CPB, where the circumflex artery and sufficient de-airing of the left ventricle should be evaluated, and the post CPB period. The post CPB TEE examination should identify: (I) residual mitral regurgitation; (II) possible mitral stenosis; and (III) potential complications of the surgical mitral valve, i.e., systolic anterior motion (SAM), distortion of the circumflex artery, new onset of aortic regurgitation, aortic dissection etc.
Pre-operative TEE examination
Evaluation of the mitral valve apparatus
To evaluate the mitral valve apparatus with 2D TEE, the following standard views are necessary:
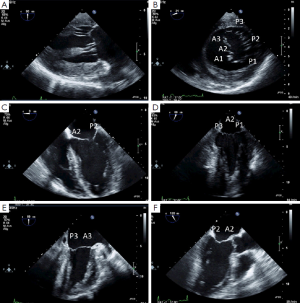
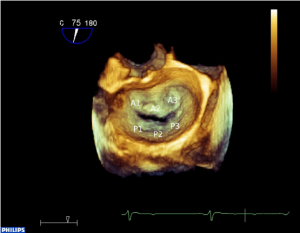
In the transgastric two-chamber view, the inner dimension of the left ventricle in systole and diastole should be measured (9). This view also allows good visualization of the subvalvular mitral valve apparatus (i.e., papillary muscles and chordae, Figure 1A). The transgastric basal short axis view allows planimetry of the mitral valve opening area and visualization of all segments including both commmissures (see Figure 1B). In the midesophageal four chamber-, mitral commissural-, two chamber- and long axis views, different segments of the mitral valve leaflets can be seen (see Figure 1C-F). Because the phased-array technology of 2D TEE provides only small cross-sectional images of the heart, mental three-dimensional reconstruction is necessary to get a complete impression of the morphology and pathology of the whole mitral valve apparatus. New matrix transducer technology allows real-time three-dimensional TEE (RT 3D TEE) in the perioperative setting (10) (Figure 2).
Function of the mitral valve leaflets
According to the Carpentier classification, the motion of the mitral valve leaflets can be normal, excessive or restrictive (11). In selecting the type of surgical mitral valve repair, it is essential to identify the pathological segments. The advantage of RT 3D TEE over 2D TEE in localizing diseased segments, according to the Carpentier classification, is still a matter of debate. However, RT 3D TEE is superior in detecting some pathologies of the MV (12-14), especially involving the commissures. The agreement between preoperative echocardiographic findings and direct surgical inspection of the MV ranges between 88-100% (15), assuming that surgical inspection is the “gold standard”.
Severity of mitral regurgitation
In common practice, there are three methods to quantify the severity of mitral valve regurgitation: the calculation of effective regurgitation orifice area (EROA) by using proximal isovelocity surface area (PISA), the measurement of the vena contracta width and the interrogation of pulmonary vein flow.
The calculated EROA, as the most robust parameter of all three, is recommended whenever feasible. According to the guidelines of the European Association of Echocardiography (EAE), mitral regurgitation is classified as mild (EROA <20 mm2), moderate (EROA 20-39 mm2), and severe (EROA >40 mm2) (2).
Vena contracta is the narrowest width, with the highest velocity of a flow jet measured at the atrial side of the leaflet tips. A vena contracta width <3 mm denotes mild and >7 mm is specific for severe mitral regurgitation. Intermediate values roughly correlate with moderate mitral regurgitation. In this case, another quantitative method should be used for confirmation. Vena contracta works well for central and eccentric jets, but it is difficult to apply in multiple mitral regurgitation jets.
For both EROA and Vena contracta methods, geometric assumptions are required that can limit the clinical application. Most of the regurgitation jets are not circular in shape (16). The limitation of these two commonly used 2D measurements may be overcome with 3D color Doppler echocardiography and the planimetry of the regurgitant orifice area (17).
General anesthesia in patients undergoing mitral valve repair results in an underestimation of the severity of mitral regurgitation due to altered loading conditions (18,19). Adjusting the baseline shift of the Nyquist limit can partially compensate for that effect (19). Therefore an intraoperative graduation of the mitral regurgitation before repair is essential to act as a baseline for the postoperative evaluation of a residual mitral regurgitation.
In normal pulmonary venous flow, the S-wave is larger than the D-wave with both waves in the same direction from baseline. With increasing severity of mitral regurgitation, systolic velocity gradually decreases and even reverses in severe mitral regurgitation (Figure 3). Normally, the pulmonary flow is measured in the left upper pulmonary vein by TEE. In the presence of an eccentric regurgitation, jet evaluation in both left and right upper pulmonary veins is recommended.

Potential risk factors for surgical repair
Secondary SAM is a complication virtually unique to mitral valve repair. The postoperative incidence is reported to range between 1-16%. SAM can be diagnosed as minor chordal protrusion in the LVOT to life-threatening LVOT obstruction with mitral regurgitation. In the preoperative TEE (ME AV SAX view) structural and geometric factors like the anterior to posterior leaflet height ratio (<1.4), the absolute height of the posterior leaflet (>1.5 cm) and the minimum distance from the coaptation point to the septum (C-Sept, <2.5 cm) can be used as predictors of LVOT obstruction and SAM (20).
Distortion of the circumflex artery, due to sutures necessary to fix the annuloplasty ring during mitral valve repair occurs in up to 1.8% of patients (21). With TEE, it is possible to visualize the circumflex artery in most of the patients undergoing mitral valve repair (22). The comparison between pre- and postoperative assessment of the circumflex artery helps to identify patients with compromised circumflex artery as early as possible after mitral valve repair and should therefore be part of the routine examination (23).
Correct placement of the cannula
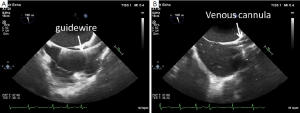
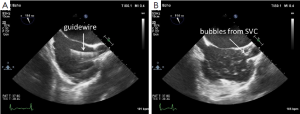
Correct positioning of the guide-wire (Figure 4A) and subsequently of the venous cannula inserted from the groin is usually performed in the midesophageal bicaval view. If no additional cannula for the superior vena cava is used, optimal position is with the tip at least 2-3 cm in the superior vena cava (Figure 4B). With a second cannula in the superior vena cava inserted from the right internal jugular vein, the femoral cannula should be positioned in the inferior vena cava. Before insertion of the jugular venous cannula, the position of the guide wire in the right atrium should be verified to avoid vascular complications (Figure 5A). After insertion of the cannula, a “bubble test” should be performed to ensure endovascular positioning of the cannula (Figure 5B). Before insertion of the arterial cannula into the femoral artery, the guide-wire in the descending aorta should also be visualized, to avoid vascular complications. For port-access minimally invasive mitral valve surgery, the position of the endoaortic balloon in the ascending aorta has to be verified in the midesophageal ascending aortic long axis view (24,25).
Post-operative TEE examination
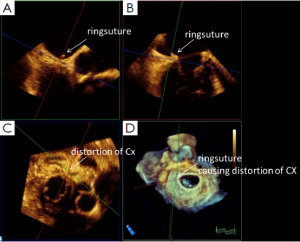
Immediately after release of the cross clamp, visualization of the circumflex artery allows early detection of a compromised circumflex artery when compared with the pre-operative TEE examination (26). With RT 3D TEE, the suture causing distortion of the circumflex artery can be identified (Figure 6).
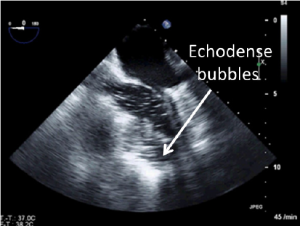
Before removal of the surgical vents, one has to check for complete de-airing of the left ventricle to avoid air embolism in the coronary arteries. Intracavitary air is clearly visualized as echodense bubbles in the midesophageal views (Figure 7).
Residual mitral regurgitation after repair should be evaluated only after complete weaning from bypass. The same methods for graduation can be used as in the pre-operative evaluation.
Post-operative mitral stenosis
Mitral stenosis after repair occurs in less than 2% of patients. A pressure gradient of <7 mmHg measured with continuous wave Doppler is associated with significant mitral stenosis. Planimetry of the mitral valve opening area should be performed in the transgastric basal short axis view when using 2D TEE or even better with RT 3D TEE.
Dynamic left ventricular outflow obstruction with accompanying SAM is best visualized in the ME LAX and five-chamber views. Continuous wave Doppler signal of the left ventricular outflow tract shows a characteristic dagger-shaped form because the peak pressure gradient occurs in late systole.
Finally, excluding new onset of aortic regurgitation due to a captured non-coronary cusp of the aortic valve by annuloplasty sutures, aortic dissection (27) and ventricular rupture (28) should be part of the post-operative TEE examination.
Future perspective
Real-time three-dimensional TEE offers anatomical visualization of the mitral valve apparatus, fundamental for echocardiographic guidance and virtual surgical planning. New surgical interventional (i.e., MitralClip®) and surgical off-pump techniques (i.e., Neochord®) completely rely on echocardiographic guidance (29,30). The results of studies predicting proper annuloplasty ring size using RT 3D TEE and special software are encouraging (31,32).
Conclusions
There is no doubt that the diagnostic value of perioperative TEE has improved the safety of MV surgery. 2D and RT 3D TEE are complementary in diagnosing complex pathology of the MV and assessing MV repair. As we embrace new technologies, a collaborative approach among the “heart team” assumes even greater importance in ensuring good postoperative outcomes. Finally, while many issues must be overcome in the management of patients with MV pathology, as Karl Popper famously stated, “When we do research, we never apprehend the truth, we merely reduce the level of our error.”
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Matsumoto M, Oka Y, Lin YT, et al. Transesophageal echocardiography; for assessing ventricular performance. N Y State J Med 1979;79:19-21. [PubMed]
- Lancellotti P, Moura L, Pierard LA, et al. European association of echocardiography recommendations for the assessment of valvular regurgitation. Part 2: mitral and tricuspid regurgitation (native valve disease). Eur J Echocardiogr 2010;11:307-32. [PubMed]
- Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC), European Association for Cardio-Thoracic Surgery (EACTS), Vahanian A, et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 2012;33:2451-96. [PubMed]
- Shanewise JS, Cheung AT, Aronson S, et al. ASE/SCA guidelines for performing a comprehensive intraoperative multiplane transesophageal echocardiography examination: recommendations of the American Society of Echocardiography Council for Intraoperative Echocardiography and the Society of Cardiovascular Anesthesiologists Task Force for Certification in Perioperative Transesophageal Echocardiography. Anesth Analg 1999;89:870-84. [PubMed]
- Eltzschig HK, Rosenberger P, Loffler M, et al. Impact of intraoperative transesophageal echocardiography on surgical decisions in 12,566 patients undergoing cardiac surgery. Ann Thorac Surg 2008;85:845-52. [PubMed]
- Klein AA, Snell A, Nashef SA, et al. The impact of intra-operative transoesophageal echocardiography on cardiac surgical practice. Anaesthesia 2009;64:947-52. [PubMed]
- Skinner HJ, Mahmoud A, Uddin A, et al. An investigation into the causes of unexpected intra-operative transoesophageal echocardiography findings. Anaesthesia 2012;67:355-60. [PubMed]
- Buck T, Kortmann K, Plicht B, et al. Critical importance of unsuspected findings detected by intraoperative transesophageal echocardiography for decision making during cardiac surgery. Clin Res Cardiol 2013;102:351-9. [PubMed]
- Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005;18:1440-63. [PubMed]
- Lang RM, Badano LP, Tsang W, et al. EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. J Am Soc Echocardiogr 2012;25:3-46. [PubMed]
- Carpentier A. Cardiac valve surgery--the “French correction”. J Thorac Cardiovasc Surg 1983;86:323-37. [PubMed]
- Grewal J, Mankad S, Freeman WK, et al. Real-time three-dimensional transesophageal echocardiography in the intraoperative assessment of mitral valve disease. J Am Soc Echocardiogr 2009;22:34-41. [PubMed]
- Mukherjee C, Tschernich H, Kaisers UX, et al. Real-time three-dimensional echocardiographic assessment of mitral valve: is it really superior to 2D transesophageal echocardiography? Ann Card Anaesth 2011;14:91-6. [PubMed]
- Manda J, Kesanolla SK, Hsuing MC, et al. Comparison of real time two-dimensional with live/real time three-dimensional transesophageal echocardiography in the evaluation of mitral valve prolapse and chordae rupture. Echocardiography 2008;25:1131-7. [PubMed]
- Moustafa SE, Chandrasekaran K, Khandheria B, et al. Real-time three-dimensional transesophageal echocardiography assessment of the mitral valve: perioperative advantages and game-changing findings. J Heart Valve Dis 2011;20:114-22. [PubMed]
- Kahlert P, Plicht B, Schenk IM, et al. Direct assessment of size and shape of noncircular vena contracta area in functional versus organic mitral regurgitation using real-time three-dimensional echocardiography. J Am Soc Echocardiogr 2008;21:912-21. [PubMed]
- Altiok E, Hamada S, van Hall S, et al. Comparison of direct planimetry of mitral valve regurgitation orifice area by three-dimensional transesophageal echocardiography to effective regurgitant orifice area obtained by proximal flow convergence method and vena contracta area determined by color Doppler echocardiography. Am J Cardiol 2011;107:452-8. [PubMed]
- Grewal KS, Malkowski MJ, Piracha AR, et al. Effect of general anesthesia on the severity of mitral regurgitation by transesophageal echocardiography. Am J Cardiol 2000;85:199-203. [PubMed]
- Heß H, Eibel S, Mukherjee C, et al. Quantification of mitral valve regurgitation with color flow Doppler using baseline shift. Int J Cardiovasc Imaging 2013;29:267-74. [PubMed]
- Maslow AD, Regan MM, Haering JM, et al. Echocardiographic predictors of left ventricular outflow tract obstruction and systolic anterior motion of the mitral valve after mitral valve reconstruction for myxomatous valve disease. J Am Coll Cardiol 1999;34:2096-104. [PubMed]
- Aybek T, Risteski P, Miskovic A, et al. Seven years’ experience with suture annuloplasty for mitral valve repair. J Thorac Cardiovasc Surg 2006;131:99-106. [PubMed]
- Ender J, Gummert J, Fassl J, et al. Ligation or distortion of the right circumflex artery during minimal invasive mitral valve repair detected by transesophageal echocardiography. J Am Soc Echocardiogr 2008;21:408.e4-5.
- Ender J, Singh R, Nakahira J, et al. Echo didactic: visualization of the circumflex artery in the perioperative setting with transesophageal echocardiography. Anesth Analg 2012;115:22-6. [PubMed]
- Falk V, Walther T, Diegeler A, et al. Echocardiographic monitoring of minimally invasive mitral valve surgery using an endoaortic clamp. J Heart Valve Dis 1996;5:630-7. [PubMed]
- Aybek T, Doss M, Abdel-Rahman U, et al. Echocardiographic assessment in minimally invasive mitral valve surgery. Med Sci Monit 2005;11:MT27-32. [PubMed]
- Ender J, Singh R, Nakahira J, et al. Echo didactic: visualization of the circumflex artery in the perioperative setting with transesophageal echocardiography. Anesth Analg 2012;115:22-6. [PubMed]
- Williams ML, Sheng S, Gammie JS, et al. Clark Award. Aortic dissection as a complication of cardiac surgery: report from the Society of Thoracic Surgeons database. Ann Thorac Surg 2010;90:1812-6; discussion 1816-7.
- Deniz H, Sokullu O, Sanioglu S, et al. Risk factors for posterior ventricular rupture after mitral valve replacement: results of 2560 patients. Eur J Cardiothorac Surg 2008;34:780-4. [PubMed]
- Faletra FF, Pedrazzini G, Pasotti E, et al. Role of real-time three dimensional transoesophageal echocardiography as guidance imaging modality during catheter based edge-to-edge mitral valve repair. Heart 2013;99:1204-15. [PubMed]
- Seeburger J, Borger MA, Tschernich H, et al. Transapical beating heart mitral valve repair. Circ Cardiovasc Interv 2010;3:611-2. [PubMed]
- Ender J, Koncar-Zeh J, Mukherjee C, et al. Value of augmented reality-enhanced transesophageal echocardiography (TEE) for determining optimal annuloplasty ring size during mitral valve repair. Ann Thorac Surg 2008;86:1473-8. [PubMed]
- Ender J, Eibel S, Mukherjee C, et al. Prediction of the annuloplasty ring size in patients undergoing mitral valve repair using real-time three-dimensional transoesophageal echocardiography. Eur J Echocardiogr 2011;12:445-53. [PubMed]