Improvement of left atrial function and left atrial reverse remodeling after surgical treatment of atrial fibrillation
Introduction
Two-dimensional speckle tracking echocardiography (2D-STE) is an emerging technology for the evaluation of left atrial (LA) function. This technique is feasible, overcomes the limitations of tissue Doppler imaging and has been demonstrated to accurately assess atrial function during the different phases of the cardiac cycle (1).
Atrial structural and electrical remodeling are the hallmarks of atrial fibrillation (AF), and left atrial reverse remodeling (LARR) with recovery of atrial function are considered important targets of any AF treatment. While reverse remodeling has been demonstrated to occur after catheter ablation irrespective of the paroxysmal or persistent nature of AF (2), little is known about LVRR and atrial function in response to successful surgical AF ablation.
Therefore, we evaluated LA function and reverse remodeling after restoration of sinus rhythm following minimally invasive atrial fibrillation surgery (MIAFS).
Methods
Thirty-three patients with paroxysmal AF undergoing radiofrequency MIAFS at our Institution (Department of Cardiothoracic Surgery, University Hospital Maastricht, Maastricht, The Netherlands) from 2007 to 2011 were the subject of the present study. Twenty age- and gender-matched healthy adults were controls. Details of definitions, inclusion criteria and operative technique have been described in our previous work (3).
All patients were followed-up according to the Heart Rhythm Society/European Heart Rhythm Association/European Cardiac Arrhythmia Society (HRS/EHRA/ECA) expert consensus statement on catheter and surgical ablation of AF. Seven-day Holter Monitoring (HM) was performed at 3 months, 6 months and 1 year and yearly thereafter. All patients reached 1-year follow up. Patient characteristics are listed in Table 1.
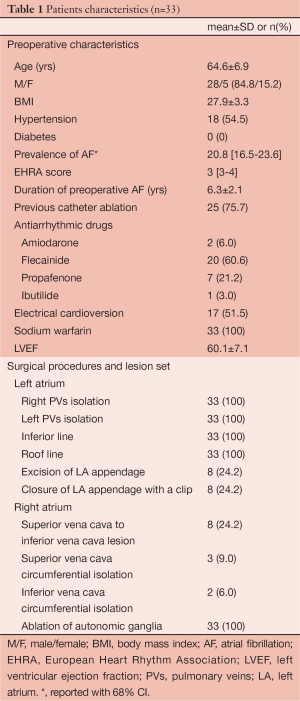
Full table
Echocardiography
Echocardiography was performed preoperatively and at 12-month follow-up by two experienced echocardiographers (F.L. and C.M.R.) using a commercially available system (Philips iE33; Philips Medical Systems, Eindhoven, The Netherlands). All parameters were analyzed off-line using Xcelera software (Philips Medical Systems). In the apical 4-chamber view, the LA maximum volume (LAMAX) and LA minimum volume (LAMIN) were measured, the former at the end of left ventricular (LV) systole just before the opening of the mitral valve and the latter at the end of LV diastole just after the closure of the mitral valve. The LA emptying fraction was calculated as follows: [(LAMAX – LAMIN)/LAMAX] ×100. The LA maximum volume was also measured using the biplane area-length method and indexed to the body surface area (LA volume index, LAVI). The LARR was defined as a reduction in the LAVI ≥15%.
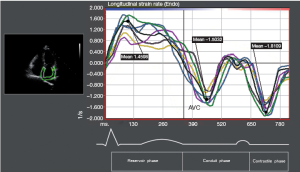
Longitudinal LA strain was computed using speckle-tracking echocardiography (2-dimensional cardiac performance analysis; TomTec Imaging Systems, Munich, Germany). The data from a total of 12 LA segments (annular, mid and superior segments along the septal, lateral, anterior and inferior LA walls using apical 4-chamber and 2-chamber images) were averaged to determine the global LA peak systolic strain (εP) during LV ejection (LA reservoir phase). The peak systolic strain rate (SRP) was measured during LV ejection (LA reservoir phase). The peak early diastolic strain rate (SRE) was measured during LV early diastole (LA conduit phase) and peak late diastolic strain rate (SRA) was measured during LV diastole occurring after the P wave (active contraction phase).
Statistical methods
Statistical analysis was performed using SPSS release 12.0 (SPSS, Chicago, IL, USA). Parametric values were expressed as the mean ± standard deviation, nonparametric values as the median and interquartile range (IQR), and categorical variables as percentages. Normally distributed variables were compared using a paired t-test and the Fisher and Mc Nemar tests were used to compare unpaired and paired categorical data, respectively. Interobserver and intraobserver variability were examined using Pearson bivariate correlation, the result of which was satisfactory (Table 2).
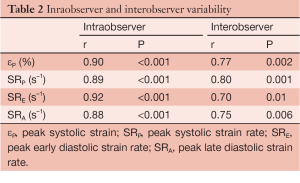
Full table
Results
At baseline (Figure 1) there was an increase in LAMAX (Pvs. controls), LAMIN (Pvs. controls) and biplane LAVI (P=0.02 vs. controls). All these volumes were significantly reduced at 12-month postoperative control (LAMAX P=0.03, LAMIN P=0.03, LAVI P=0.04). According to the cutoff value (≥15% decrease in LAVI), LARR occurred in 72.7% of patients (n=24) at 12-month echocardiographic control. In addition, LAVI in non-responders decreased by 10% or more in 6 patients (18.2%), vs. 65%±13%, Pvs. baseline).
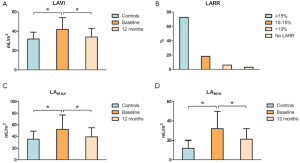
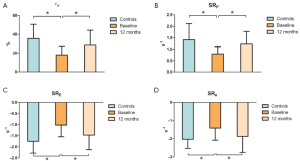
Figure 2 shows strain measurements in patients and controls. The global εP was reduced in patients with AF compared with that in the controls (PFigure 3), the SRP (PE (PA (P
Discussion
2D-STE is a no-Doppler, angle-independent technique which allows the measurement of global as well as regional atrial strain and strain rate (SR) (1).
The strain and SR curves evaluated by 2D-STE closely follow changes in atrial function during the different phases of the cardiac cycle (4). LA global function has three phases: (I) reservoir phase, during which filling proceeds from the pulmonary veins, contemporaneous with ventricular systole; during this phase (including LV isovolumic contraction, ejection and isovolumic relaxation), LA strain increases, achieving a peak at the end of LA filling from the venous district just before MV opening; (II) conduit phase, characterized by passive flow from pulmonary veins down a pressure gradient initiated by LV relaxation; during this phase LA strain decreases, showing a plateau during diastasis; (III) active contractile phase (booster pump function), during which the atrium contracts and expels blood into the LV in late diastole.
To the best of our knowledge, this is the first study exploring changes in LA structure and function after AF minimally invasive surgery using 2D-STE.
In our experience, patients preoperatively showed a significant impairment in LA compliance (reservoir function) estimated by the global εP and SRP. Indeed, both these indices increased significantly after surgery, suggesting that such an improvement in atrial compliance might have resulted from still reversible LA functional changes in these patients. The improvement in atrial compliance is also demonstrated by a significant postoperative increase in the LAEF. However, although the exact mechanism remains unclear, the impairment of atrial compliance has been demonstrated to play an important role in reversing LA enlargement and maintaining sinus rhythm during follow-up (5).
Furthermore, the baseline LA conduit function (during LV passive filling) was also significantly altered and postoperatively, we observed a significant improvement in the conduit phase as demonstrated by the enhanced SRE, values.
In addition, an important finding in our study was the significant improvement in LA pump function, demonstrated by an increase, at 12-month echocardiography, of SRA that was severely affected preoperatively. Finally, the increment in LA volume is one of the most important structural pathologic changes in AF, and reverse remodeling of the left atrium is considered an important target of AF treatment. In our experience, using the cutoff value ≥15% decrease in LAVI, LA reverse remodeling (LARR) occurred in 72.7% of patients postoperatively. This represents an important finding of our study, since the LA volume showed a strong correlation with the extent of fibrosis and it was associated with greater mortality in patients with AF (2).
Apart from the small number of patients, the main limitation of our study is the inclusion of only patients with paroxysmal AF. As is well known, these patients present with minor changes in LA tissue and muscle (substrate modification) and, therefore, a lower extent of LA remodeling. Unfortunately, to accurately study LA function with 2D-STE, it is necessary to have echocardiograms during sinus rhythm. Furthermore, in subjects with persistent or long-standing persistent AF, it is very difficult to have periods of sinus rhythm long enough to allow a sufficient number of good quality images for 2D-STE analysis.
In conclusion, even with the afore mentioned limitations, radiofrequency MIAFS resulted, in our experience, in significant LA reverse remodeling and improvement of LA function. Larger studies with a longer follow-up are necessary to confirm our findings.
Acknowledgements
We gratefully acknowledge Dr Judith Wilson for the English revision of the manuscript.
Disclosure: M.L.M. is consultant/advisor for Atricure. Other co-authors have no conflict of interest.
References
- Di Salvo G, Drago M, Pacileo G, et al. Atrial function after surgical and percutaneous closure of atrial septal defect: a strain rate imaging study. J Am Soc Echocardiogr 2005;18:930-3. [PubMed]
- Kuppahally SS, Akoum N, Badger TJ, et al. Echocardiographic left atrial reverse remodeling after catheter ablation of atrial fibrillation is predicted by preablation delayed enhancement of left atrium by magnetic resonance imaging. Am Heart J 2010;160:877-84. [PubMed]
- La Meir M, Gelsomino S, Lucà F, et al. Improvement of left atrial function and left atrial reverse remodeling after minimally invasive radiofrequency ablation evaluated by 2-dimensional speckle tracking echocardiography. J Thorac Cardiovasc Surg 2013;146:72-7. [PubMed]
- Payne RM, Stone HL, Engelken EJ. Atrial function during volume loading. J Appl Physiol 1971;31:326-31. [PubMed]
- Tops LF, Delgado V, Bertini M, et al. Left atrial strain predicts reverse remodeling after catheter ablation for atrial fibrillation. J Am Coll Cardiol 2011;57:324-31. [PubMed]





