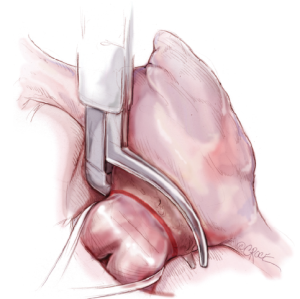
Illustrated techniques for performing the Cox-Maze IV procedure through a right mini-thoracotomy
Introduction
The first effective surgical procedure to treat atrial fibrillation (AF) was introduced by Dr James Cox in 1987 after extensive animal investigation. This procedure, now formally known as the Cox-Maze procedure, utilized a biatrial “cut-and-sew” technique in an attempt to guide the native sinus impulse to both atria and the atrioventricular (AV) node while blocking all possible macroreentrant circuits. By restoring sinus rhythm and AV synchrony while maintaining atrial transport function, the Cox-Maze procedure significantly reduced the risk of thromboembolism, stroke and hemodynamic compromise (1,2). It was designed to provide a standardized approach that would be effective in all patients. Unfortunately, the first version of the Cox-Maze procedure was complicated by late chronotropic incompetence and a high rate of pacemaker implantation (3). To address the problem, the Cox-Maze procedure underwent two further modifications, eventually resulting in the Cox-Maze III. This lesion set became the gold standard for the surgical treatment of AF (4). Despite its clinical success, however, the procedure was seldom performed due to its technical complexity and prolonged time on cardiopulmonary bypass (CPB).
In order to simplify the Cox-Maze procedure, our group replaced the majority of the incisions of the Cox-Maze III with a combination of bipolar radiofrequency (RF) and cryothermal ablation lines in a procedure termed the Cox-Maze IV, which was introduced clinically in 2002. This and the introduction of other ablation-based procedures have resulted in a dramatic increase in the number of operations performed annually for AF. In 2005, 12,737 patients were reported to the STS Database as having undergone a surgical procedure for AF, whereas one year prior the number of patients was 3,987 (5). Before 2004, the volume was so low that the operation was not reported. While exact numbers have not been published, the number of AF procedures performed annually has continued to increase over the last several years.
Several different lesion sets, including isolated pulmonary vein isolation (PVI), extended left atrial lesion sets and hybrid procedures have been performed with various results. Similarly, a variety of ablation technologies have been employed by different groups. Some of these devices have been removed from the market due to poor performance. Greater uniformity in both technique and follow-up standards (6) is needed if outcomes are to be compared. This review aims to clearly relate and illustrate proper techniques for successfully performing a Cox-Maze IV procedure, as well as to provide insight into our choice of ablation technology and the reasons why certain critical steps are performed.
Operative techniques
Preoperative evaluation
Patient-specific anatomical considerations, particularly left atrial substrate, may affect operative outcomes. The left atrial diameter and volume should be obtained in all patients using transthoracic echocardiography (TTE). Increases in these measurements are associated with a higher failure rate of the CMIV procedure (7). Once left atrial diameter is ≥8 cm, the failure rate is over 50%, and this may influence the decision as to whether to proceed with surgical ablation. TTE is also useful to characterize the degree and nature of concomitant valvular disease. Moreover, patients with prior failed catheter ablations should undergo a pulmonary vein (PV) protocol computed tomography (CT) scan in order to exclude PV stenosis. While rarely detected, PV stenosis would require repair if identified. Finally, coronary angiography is recommended in order to identify any existing lesions and to define the anatomy. The circumflex artery is at risk of injury during the mitral isthmus ablation in patients with a left-dominant circulation. Significant atrial fibrosis secondary to long-standing AF may also affect outcomes. However, there are not currently any validated methods available to assess this preoperatively. Delayed-enhancement cardiac magnetic resonance imaging (DE-MRI) is one promising technique that is currently under investigation (8-10).
There are also a few preoperative considerations unique to performing a Cox-Maze IV through a right mini-thoracotomy. First, a detailed history of pulmonary disease and sleep apnea should be obtained, with further evaluation undertaken if there are any perceived difficulties with performing single-lung ventilation. Second, patients at risk of vascular disease and those over 65 years of age should undergo a CT angiogram of the thoracic and abdominal aorta, iliac and femoral vessels and a carotid Doppler ultrasound to assess the quality of aortic disease and the safety of femoral cannulation.
Most patients are referred for AF correction surgery with excellent documentation of their rhythm abnormalities. However, in patients for whom this is not the case, it is recommended that the surgeon obtain prolonged Holter monitoring. Event monitors are particularly helpful in patients with paroxysmal arrhythmias to determine whether symptoms are related to arrhythmia occurrence.
Management of anti-arrhythmic and anticoagulation therapies is also usually required in the immediate preoperative period. Generally, anti-arrhythmic and rate-control medications should be continued through the morning of surgery. Patients who have been anticoagulated with warfarin are typically managed with intravenous heparin or bivalirudin in the preoperative period.
Preparation
Typically, a large bore peripheral intravenous line and an intra-arterial pressure catheter are placed in the pre-operative holding area. After induction, a 9 French introducer catheter is placed in the right internal jugular vein using ultrasound guidance. Generally, a pulmonary artery catheter (PAC) is not used since it often gets in the way of performing the right atrial lesions. If it is felt to be necessary for postoperative monitoring, the PAC is left in the superior vena cava (SVC) until the end of the case. Another strategy is to place a dual-lumen infusion catheter at the onset of the case and convert to a PAC if needed at the end of the procedure.
Although all patients undergo a TTE during preoperative assessment and planning, the left atrial appendage (LAA) is not well visualized with this modality, and, therefore, thrombus cannot be excluded with certainty. Prior to the start of the operation, the left atrium is thoroughly examined with transesophageal echocardiography (TEE) using a variety of angles to exclude thrombus in the LAA. In patients with thrombus in the LAA, the sequence of the operation is altered so that there is minimal manipulation of the heart. At this time, the atrial septum is also assessed for the presence of a patent foramen ovale, which should be repaired if present. If the patient has concomitant mitral valve dysfunction noted on preoperative transthoracic echocardiography, it is confirmed using two-dimensional, color-flow Doppler and three-dimensional modalities. The rest of the heart is then examined for signs of concomitant disease. At the conclusion of the procedure, TEE is used to confirm successful exclusion of the LAA and, when appropriate, satisfactory repair or replacement of the MV.
In the absence of specific allergies, the patient is given intravenous cefazolin and vancomycin thirty minutes prior to skin incision. Intravenous cefazolin is re-dosed after separation from CPB, and both antibiotics are continued for 48 hours postoperatively.
Positioning
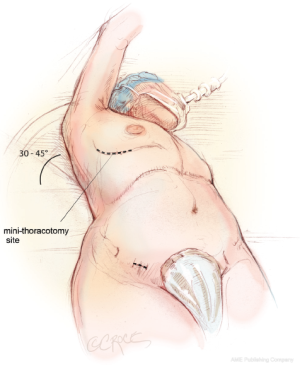
The patient is brought to the operating room and placed in the supine position on the operating room table. The patient is intubated using a regular endotracheal tube with a bronchial blocker for single-lung ventilation. In some patients, a double lumen endotracheal tube is used to achieve similar results. In preparation for a right mini-thoracotomy, the patient is positioned with the right chest elevated at approximately a 30-45° angle, with the hips as flat as possible against the operating room table (Figure 1). The right arm is brought over the patient’s head and placed in an anatomic position on an elevated arm board.
The patient is prepped circumferentially from the chest to the ankles with multiple layers of chlorhexidine gluconate/isopropyl alcohol antiseptic solution. An antimicrobial surgical drape with an iodophor impregnated adhesive is then applied to the patient’s chest, abdomen and groin.
Cannulation
In the absence of any contraindications, the right femoral vessels are used for CPB. The femoral pulse is palpated at the level of the inguinal ligament, and the location is marked on the skin (Figure 1). A 2 cm subinguinal incision is performed parallel to the skin creases, overlying the area of the femoral pulse, and the femoral artery and vein are exposed. Proximal and distal control of the vessels is obtained, and the patient is cannulated using a 25 French venous cannula and either a 17 or 19 French arterial cannula, depending on the size of the femoral artery. The arterial cannula is placed first and TEE is used to confirm placement of the guidewire in the descending thoracic aorta in order to prevent an aortic injury. The venous cannula is then advanced into the right atrium until resistance is felt. It is ultimately advanced to the SVC by palpation or TEE guidance. The patient is fully anticoagulated with heparin to obtain an ACT ≥500, and systemic anticoagulation is maintained throughout the procedure according to the standardized heparin protocol used for CPB.
Postoperative bleeding is a relatively rare event with the Cox-Maze IV (11). However, in order to further minimize this risk, the patient is routinely given intravenous tranexamic acid at a dose of 10 mg/kg prior to the initiation of CPB, with further infusion of 5 mg/kg up to a total dose of 3 gm. If the patient has other independent risk factors for bleeding, the dose of intravenous tranexamic acid is increased to as high as 30 mg/kg. In the setting of renal insufficiency or independent risk factors for thrombosis, the bolus and infusion doses are reduced or eliminated, as appropriate.
Surgical approach
When the CMIV is performed as a lone operation or as a concomitant procedure to mitral valve surgery, we favor a less invasive right mini-thoracotomy approach in the absence of any contraindications. This is the approach that we will focus on in this review. We reserve a median sternotomy for patients with peripheral vascular disease that precludes femoral cannulation, a history of previous right thoracotomy, severe left ventricular dysfunction, or chest wall deformities such as pectus excavatum.
The 5-6 cm right mini-thoracotomy incision is made over the fourth intercostal space lateral to the nipple in the mid-axillary line in men, whereas a submammary incision is used in women (Figure 1). The chest is entered through the fourth intercostal space, and a small segment of the posterior fifth rib is divided. Access to the fourth intercostal space requires tunneling to the appropriate level when a submammary incision is used. A soft tissue retractor is used to provide exposure through the mini-thoracotomy, and it is further secured to the skin using additional Ioban adhesive sheets. It is important not to use a metal retractor for rib spreading, as this increases postoperative pain.
CBP is established with the patient initially maintained at normothermia, and the right lung is deflated. A Blake drain is placed percutaneously via a stab wound on the lateral chest wall, usually overlying the right seventh intercostal space along the mid-axillary line, and positioned into the posterior pleural space. Carbon dioxide is infused into this drain throughout the entire case to prevent air embolism. If needed, a pledgeted 0 Tevdek suture is then used to retract the diaphragm and provide exposure. The stitch should be placed superficially so as to avoid liver injury, and retraction is performed by passing the suture through the incision created for placement of the Blake drain.
Initial exposure and pulmonary vein isolation (PVI)
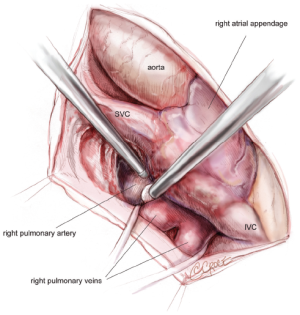
The pericardium is opened from the diaphragm to the mid-ascending aorta, with care taken to make the pericardiotomy about 2-4 cm away from the phrenic nerve in order to avoid subsequent traction injury. The heart is suspended in a pericardial cradle. A 5 mm, 30-degree endoscope is then placed through a port in the 6th intercostal space close to the posterior-axillary line. The SVC and inferior vena cava (IVC) are mobilized. The space underneath the SVC, the area between the right superior PV and the right pulmonary artery, and the oblique sinus are developed (Figure 2). It is important to bluntly divide the tissue between the posterior wall of the aorta and the anterior wall of the pulmonary artery in order to create room for the aortic cross-clamp. Dissections of the transverse sinus and Waterson’s groove (indicating the position of the interatrial septum) are also completed from below the SVC. The LAA will be visible through the transverse sinus. It must be remembered that these planes may be replaced with significant amounts of scar in patients who have had multiple previous ablations. All of these dissections are typically performed using Bovie electrocautery and an endoscopic Kittner.
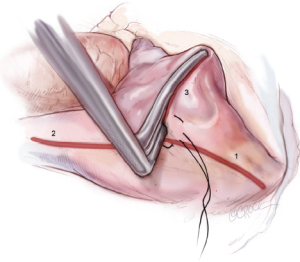
The right PVs are bluntly dissected and encircled with umbilical tape using a renal pedicle clamp. Once the exposure is complete, amiodarone is administered, and the patient is electrically cardioverted to normal sinus rhythm if necessary. Pacing thresholds are obtained from the right superior and inferior PVs using a bipolar pacing probe. The right PVs are then isolated using a bipolar RF clamp, such that a linear line of ablation surrounds as large a cuff of atrial tissue around the PVs as possible (Figure 3). Three sequential ablations are performed with slight adjustments in position, effectively forming three closely approximated concentric circles. Importantly, if the surgeon is using a non-irrigated bipolar clamp, the jaws must be cleaned of char every 2-3 ablations in order to ensure creation of transmural lesions. PV isolation is documented by pacing at 20 mA from both the right superior and inferior PVs in order to confirm exit block. Further ablations are performed as needed until exit block is obtained.
Right atrial lesion set
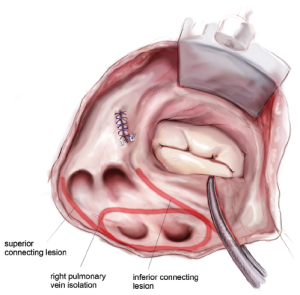
The right-sided lesions of the CMIV are created on CPB before aortic cross-clamping. A purse-string suture is placed just above the interatrial septum, midway between the SVC and IVC. Care should be taken to avoid the crista terminalis due to the thickness of the tissue in that area. A stab incision is created in the center of the purse-string, and one jaw of the bipolar RF clamp is introduced into the right atrium. Using the clamp, ablation lines are performed up onto the SVC, down onto the IVC, and across the right atrial free wall toward the AV groove near the acute margin of the heart (Figure 4). The IVC ablation line should travel as far as possible onto the IVC, and the ablation line onto the SVC should be performed as lateral/posterior as possible to minimize risk of injury to the sinus node complex. For each of these lines and all subsequent lines created using the bipolar RF clamp, three discrete ablations are performed with slight adjustments in clamp position in order to ensure isolation.
From the superior aspect of the right atrial free wall ablation line, a second purse-string suture is placed near the AV groove, being careful to avoid ensnaring the right coronary artery. This purse-string should be at the end of the prior right atrial free wall ablation in order to connect that lesion with the next. Placement of this purse-string is facilitated by allowing volume to temporarily fill the heart, which pushes the atrium towards the surgeon. A stab incision is then made in the center of the purse-string, and a 3-cm reusable linear cryoprobe is used to create an endocardial cryoablation down to the tricuspid valve annulus at the 1 o’clock position (Figure 5). The cryoprobe should be palpated in the right ventricle prior to freezing, and further confirmation that placement is correct is obtained when the beating heart deflects the probe. Cryoablations are all performed at –60 °C for three minutes each.
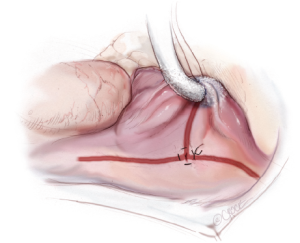
A third purse-string suture is placed at the base of the right atrial appendage. One jaw of the bipolar RF clamp is introduced through a stab incision created in the center of the purse-string, and a right atrial free wall ablation is created for several centimeters down toward the SVC (Figure 6). At least two centimeters should be left between the end of this ablation line and the SVC line in order to avoid the sinus node. The linear cryoprobe is then placed into the right atrium and used to create a second endocardial cryoablation down to the tricuspid annulus at the 11 o’clock position (Figure 7).
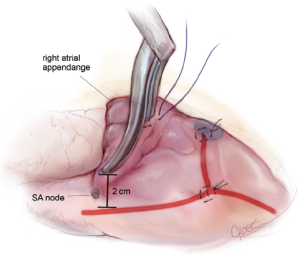
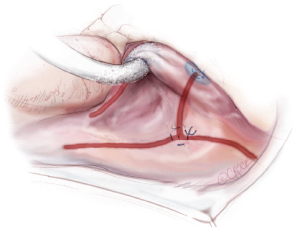
A transthoracic cross-clamp is then positioned via a stab wound in the right lateral chest wall. An aortic purse-string is placed, and a catheter for the administration of antegrade cardioplegia and aortic root venting is inserted into the proximal ascending aorta. The aorta is then cross-clamped, and the patient is cooled to 34 °C. Cold blood cardioplegia is given in an antegrade fashion to obtain myocardial protection, and additional doses of cardioplegia are administered at regular intervals throughout the remainder of the case.
Left atrial lesion set
A left atrial lift system is positioned through a stab wound in the anterior chest wall at the 4th intercostal space. It is necessary to create this stab wound under direct visualization in order to prevent injury to the right internal mammary artery. Once placed, the left atrium is opened, and the lift system is used to expose the MV and the posterior left atrium (Figure 8). The LAA is oversewn in two layers using a pledgeted, running 4-0 non-absorbable, monofilament polypropylene suture placed from the endocardial surface (Figure 9).
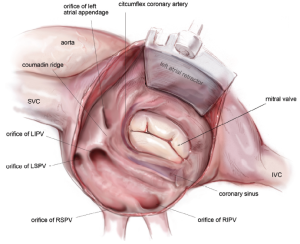
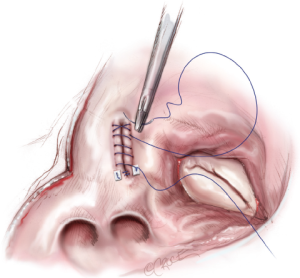
Complete posterior LA isolation is achieved by completing a “box” lesion set. From the inferior aspect of the left atriotomy, the bipolar RF clamp is used to create an ablation line across the floor of the left atrium towards the orifice of left inferior PV (Figure 10). From the superior aspect of the left atriotomy, the bipolar RF clamp is used to create another ablation line across the roof of the left atrium towards the left superior PV (Figure 10).
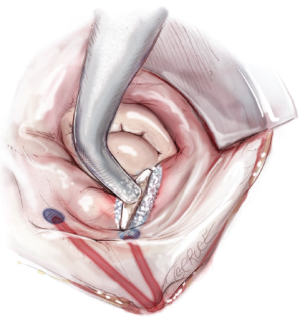
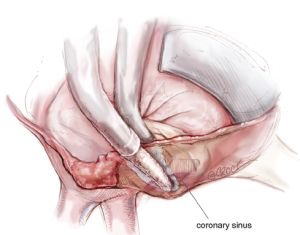
The next step is completion of the mitral isthmus line. In order to ablate the complex anatomy of the mitral isthmus, it is necessary to use a combination of bipolar RF and cryothermal energy. The bipolar RF clamp is unable to create a lesion all the way to the mitral annulus because of the thickness of the AV groove in that area. Therefore, the bipolar RF clamp is used from the inferior aspect of the left atriotomy to create an ablation line across the floor of the left atrium, down towards the MV annulus and across the coronary sinus when possible (Figure 10). In order to connect the bipolar ablation line to the annulus, it is usually necessary to bridge a 1-2 cm gap. The end of the bipolar ablation line is marked with methylene blue to provide a starting point for further ablations down to the annulus (Figure 11). We prefer to perform an endocardial cryoablation with a T-shaped cryoprobe (Figure 11) because cryoablation preserves the fibrous skeleton of the heart, making it ideal for ablation near valvular tissue. Of note, a lap pad is placed underneath the shaft of the cryoprobe during this ablation because frost forming along the shaft of the device has potential to injure the phrenic nerve. The coronary sinus must be ablated in line with the isthmus lesion in order to ensure a complete line of block. We typically accomplish this with an epicardial cryoablation overlying the coronary sinus that is performed with a linear probe (Figure 12). The cryoprobe that was used to ablate the annulus is left in place during the coronary sinus ablation so that tissue can be compressed between the shaft of the endocardial probe and the active portion of the epicardial probe. This ensures that a full thickness ablation is achieved. The bipolar clamp is not always successful in ablating the full thickness of the coronary sinus due to the anatomic variations in this structure and the thickness of the AV groove. Moreover, it is not possible to ablate the coronary sinus from the endocardial surface with a monopolar device. Of note, there is a risk to the circumflex artery when creating the left atrial isthmus lesion at the mitral annulus if patients have a left-dominant system. If a patient has a right-dominant system, the isthmus line is generally directed towards the junction of the P2 and P3 scallops of the posterior leaflet, but in patients with a left-dominant system, injury can be avoided by directing the ablation towards the posteromedial commissure.
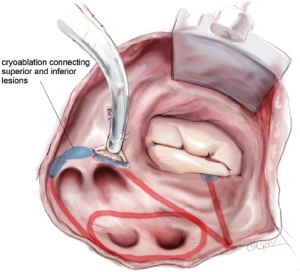
The two ablation lines down towards the left superior and inferior PVs are connected with a sequence of endocardial cryoablations created with a T-shaped cryoprobe positioned behind the left PVs and along the lateral ridge (Figure 13). This ensures complete isolation of the four PVs and the posterior left atrium. If needed, a linear cryoprobe is used from the epicardial surface to ensure ablation of all tissue.
The left atriotomy is then closed with a pledgeted, running 4-0 non-absorbable monofilament polypropylene suture. The aorta is unclamped and, after rewarming, the heart is examined with TEE for retained air, as well as ventricular and valvular function. The patient is subsequently weaned from CPB.
Closure
At the conclusion of the procedure, epicardial pacing wires are routinely placed on the right atrium and ventricle and brought out percutaneously below the fourth intercostal space. The Blake drain placed at the beginning of the case is left in the pleural space for drainage. A second drainage tube is placed through the incision previously used for the endoscope port and positioned inside the pericardium.
The femoral arterial and venous cannulas are removed and a Doppler flow probe is used to demonstrate flow in the distal femoral artery. The groin wound is irrigated with copious amounts of antibiotic-containing saline solution and meticulous hemostasis is obtained. The incision is closed in layers and a sterile dressing is applied.
Attention is then turned to the right mini-thoracotomy incision. The chest is irrigated with copious amounts of antibiotic-containing saline solution. Meticulous hemostasis is obtained, and the pericardium is loosely reapproximated with heavy silk suture. The ribs are reapproximated with 2-3 pericostal sutures. A drill is used to puncture the lower rib and the pericostal sutures are placed through the holes. This prevents entrapment of the intercostal nerve by the pericostal sutures and reduces postoperative pain. The fascia, subcutaneous tissues and skin are closed in layers. After skin closure, a sterile dressing is applied.
Median sternotomy approach
A median sternotomy may be performed if a right mini-thoracotomy is contraindicated or if the patient requires concomitant surgery that cannot be performed through a right mini-thoracotomy. A few differences should be noted in this approach. First, pacing thresholds and measurements to confirm exit block are taken from all four PVs due to ready access to both the left and right sided PVs. Second, PVI is performed on both the right and left side using a bipolar clamp instead of using a combination of cryoablation and bipolar RF ablation to complete the left sided PVI, as described above. Third, the sequence of ablations is altered. The ablation to the right atrial free wall is performed through a purse-string at the base of the right atrial appendage at the beginning of the right atrial lesion set, and the remaining purse-string sutures are replaced with a vertical atriotomy that is created from just above the interatrial septum to the AV groove near the free margin of the heart. This atriotomy should be at least 2 cm from the first free wall ablation. Through this incision, a bipolar RF clamp is used to create ablation lines up the SVC and down the IVC, and a linear cryoprobe is used to create an endocardial ablation to the tricuspid valve at the 2 o’clock position. Finally, the LAA is typically amputated instead of oversewn.
Postoperative management
One of the unique considerations in the postoperative period is rhythm management. After the CMIV, the p-wave amplitude is often small, but atrial capture is not frequently a problem. The pacemaker is set at a rate between 80 to 100 beats per minute. However, if there is first- or second-degree AV block, AV sequential pacing (DDD mode) may be required. Most patients have junctional rhythms immediately following a CMIV procedure. This has been attributed to the fact that the CMIV lesion set denervates the sinus node. This usually resolves in the first several postoperative days. Prior to resolution, however, patients should be paced in order to restore AV synchrony. Anti-arrhythmic medications should not be initiated until sinus rhythm is achieved, particularly in patients with bradyarrhythmias. This practice has evolved over the years away from more liberal use of anti-arrhythmic drugs following surgery in an attempt to minimize the incidence of postoperative permanent pacemaker insertion. Currently, only 5% of patients require a permanent pacemaker following a lone Cox-Maze IV, but this number increases with age (12). In general, sinus node function fails to recover in these patients.
Another significant consideration is management of early atrial tachyarrhythmias. In our experience, almost half of patients will experience these, although they are usually transient and frequently resolve over the first postoperative month. Stable atrial arrhytmias are managed with attempted pharmacologic cardioversion and rate-control, and, if persistent, elective direct current cardioversion is performed. Amiodarone is usually continued for the first two postoperative months. The QT interval on the EKG should be carefully followed in patients receiving amiodarone, and the drug should be discontinued if the QT interval is greater than or equal to 550 msec.
Warfarin is initiated several days after surgery, once a stable sinus rhythm has been achieved and the temporary pacing wires have been removed. It is continued for three months in all patients without contraindications to systemic anticoagulation. However, given the known risks of warfarin, we recommend discontinuation after this period if the patient has no evidence of AF, has discontinued antiarrhytmic medications, does not manifest atrial stasis on echocardiogram, and is without another indication for systemic anticoagulation. This can be done without increasing stroke risk in this population (13).
Comments
Results obtained with a Cox-Maze IV lesion set have been excellent and comparable to those obtained with the “cut-and-sew” Cox-Maze III operation (14). A prospective, single-center study from our institution followed 100 consecutive patients undergoing a lone Cox-Maze IV procedure and reported postoperative freedom from AF of 90% at both 12 and 24 months with postoperative freedom from AF and anti-arrhythmic drugs of 82% and 84% at these same time points (11). Results were similar for patients undergoing concomitant cardiac surgery (7). These results were obtained despite the fact that some patients included in this study had complex disease substrate and risk factors that would have precluded durable success with approaches such as catheter ablation. Forty percent of patients had previously failed catheter ablation, and over 60% of patients had long-standing persistent AF. Average LA diameters were 4.7±1.1 cm for lone maze patients to 5.5±1.2 cm for concomitant surgery patients.
Success of this operation is dependent on both the lesion set and the ablation technology employed. While controversial, a meta-analysis of the published literature demonstrated that biatrial lesion sets result in a significantly higher 1-year freedom from AF when compared with LA lesion sets alone (89% vs. 76%, P=0.001) (15). Moreover, we have previously demonstrated that patients who underwent a “box” lesion set had a higher freedom from AF (96% vs. 86%) and a higher freedom from AF off anti-arrhythmic drugs (79% vs. 47%) at one year compared to patients who underwent PV isolation alone or PV isolation plus a single connecting lesion. Finally, if the mitral isthmus line is either incomplete or omitted, patients are at risk for the development of late atrial flutter (16,17).
Choice of ablation technology is also critical and it should be based on the ability of each device to safely and effectively create transmural lesions without damaging important nearby structures. We have shown that gaps in ablation lines as small as 1.1 mm are still capable of propagating AF (18). Transmurality of lesions varies depending on the technology used, but animal studies have demonstrated that bipolar RF clamps and cryoablation produce transmural lesions approaching 100% of the time. Moreover, cryothermal ablation is safe around valvular tissue. The sinus node artery typically arises off of the right coronary artery and, while its course varies, it crosses the superior posterior border of the interatrial septum in 54% of cases (19). In 34-56% of cases it may alternatively arise from the left circumflex artery (19,20). Care should be taken to avoid injury to this structure. However, we have shown that bipolar RF ablation clamps do not cause significant coronary stenoses or occlude the microcirculation. In one study from our laboratory, purposeful lesions across the coronary arteries did not result in significant stenoses or thromboses, as evidenced by postoperative cardiac MRI and histology (21). We have further demonstrated via injection of lissamine green dye injection into an isolated LA preparation, that bipolar clamps do not cause any perfusion abnormalities in the microcirculation (22). These findings are supported clinically by the fact that complete isolation of the LAA across the base does not result in appendage ischemia, only electrical isolation. It is still possible that these devices create late endothelial injury.
The choice of some centers to use monopolar RF ablation technology may result in a higher incidence of nontransmural lesions and resultant higher failure rates. Moreover, while some of the cryothermal ablations could be replaced with a monopolar RF device, it would not be possible to complete the lesion set using this right mini-thoracotomy approach with just bipolar and unipolar RF technology.
In conclusion, it has been the development of ablation technology that has made this procedure both approachable in a minimally invasive fashion and accessible to the majority of surgeons. Future advances in diagnostic technologies that can precisely identify the arrhythmogenic substrate may allow us to tailor lesion sets to the pathophysiology of individual patients. At present, however, we advocate for standardization of the approach to treat AF, as well as standardization of follow-up methodology so that future advances can be appropriately compared to current standards.
Acknowledgements
Ralph J. Damiano Jr receives research grants and educational funding from AtriCure, Estech and Edwards. Ralph J. Damiano Jr also receives consultant fees from AtriCure and Medtronic.
Disclosure: The authors declare no conflict of interest.
References
- Cox JL, Ad N, Palazzo T. Impact of the maze procedure on the stroke rate in patients with atrial fibrillation. J Thorac Cardiovasc Surg 1999;118:833-40. [PubMed]
- Feinberg MS, Waggoner AD, Kater KM, et al. Restoration of atrial function after the maze procedure for patients with atrial fibrillation. Assessment by Doppler echocardiography. Circulation 1994;90:II285-92. [PubMed]
- Cox JL, Schuessler RB, Lappas DG, et al. An 8 1/2-year clinical experience with surgery for atrial fibrillation. Ann Surg 1996;224:267-73; discussion 273-5. [PubMed]
- Prasad SM, Maniar HS, Camillo CJ, et al. The Cox maze III procedure for atrial fibrillation: long-term efficacy in patients undergoing lone versus concomitant procedures. J Thorac Cardiovasc Surg 2003;126:1822-8. [PubMed]
- Gammie JS, Haddad M, Milford-Beland S, et al. Atrial fibrillation correction surgery: lessons from the Society of Thoracic Surgeons National Cardiac Database. Ann Thorac Surg 2008;85:909-14. [PubMed]
- Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm 2012;9:632-696.e21.
- Damiano RJ Jr, Schwartz FH, Bailey MS, et al. The Cox maze IV procedure: predictors of late recurrence. J Thorac Cardiovasc Surg 2011;141:113-21. [PubMed]
- Akoum N, Daccarett M, McGann C, et al. Atrial fibrosis helps select the appropriate patient and strategy in catheter ablation of atrial fibrillation: a DE-MRI guided approach. J Cardiovasc Electrophysiol 2011;22:16-22. [PubMed]
- Kuppahally SS, Akoum N, Burgon NS, et al. Left atrial strain and strain rate in patients with paroxysmal and persistent atrial fibrillation: relationship to left atrial structural remodeling detected by delayed-enhancement MRI. Circ Cardiovasc Imaging 2010;3:231-9. [PubMed]
- Oakes RS, Badger TJ, Kholmovski EG, et al. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation 2009;119:1758-67. [PubMed]
- Weimar T, Bailey MS, Watanabe Y, et al. The Cox-maze IV procedure for lone atrial fibrillation: a single center experience in 100 consecutive patients. J Interv Card Electrophysiol 2011;31:47-54. [PubMed]
- Robertson JO, Cuculich PS, Saint LL, et al. Predictors and risk of pacemaker implantation after the Cox-Maze IV procedure. Ann Thorac Surg 2013;95:2015-20; disussion 2020-1.
- Pet M, Robertson JO, Bailey M, et al. The impact of CHADS2 score on late stroke after the Cox maze procedure. J Thorac Cardiovasc Surg 2013;146:85-9. [PubMed]
- Lall SC, Melby SJ, Voeller RK, et al. The effect of ablation technology on surgical outcomes after the Cox-maze procedure: a propensity analysis. J Thorac Cardiovasc Surg 2007;133:389-96. [PubMed]
- Barnett SD, Ad N. Surgical ablation as treatment for the elimination of atrial fibrillation: a meta-analysis. J Thorac Cardiovasc Surg 2006;131:1029-35. [PubMed]
- Gaita F, Riccardi R, Caponi D, et al. Linear cryoablation of the left atrium versus pulmonary vein cryoisolation in patients with permanent atrial fibrillation and valvular heart disease: correlation of electroanatomic mapping and long-term clinical results. Circulation 2005;111:136-42. [PubMed]
- Gillinov AM, McCarthy PM, Blackstone EH, et al. Surgical ablation of atrial fibrillation with bipolar radiofrequency as the primary modality. J Thorac Cardiovasc Surg 2005;129:1322-9. [PubMed]
- Melby SJ, Lee AM, Zierer A, et al. Atrial fibrillation propagates through gaps in ablation lines: implications for ablative treatment of atrial fibrillation. Heart Rhythm 2008;5:1296-301. [PubMed]
- Berdajs D, Patonay L, Turina MI. The clinical anatomy of the sinus node artery. Ann Thorac Surg 2003;76:732-5; discussion 735-6. [PubMed]
- Zhang ZJ, Chen K, Tang RB, et al. Impact of the origin of sinus node artery on recurrence after pulmonary vein isolation in patients with paroxysmal atrial fibrillation. Chin Med J (Engl) 2013;126:1624-9. [PubMed]
- Gaynor SL, Ishii Y, Diodato MD, et al. Successful performance of Cox-Maze procedure on beating heart using bipolar radiofrequency ablation: a feasibility study in animals. Ann Thorac Surg 2004;78:1671-7. [PubMed]
- Byrd GD, Prasad SM, Ripplinger CM, et al. Importance of geometry and refractory period in sustaining atrial fibrillation: testing the critical mass hypothesis. Circulation 2005;112:I7-13. [PubMed]