Role of segmentectomy for pulmonary metastases
Introduction
The lung is one of the most common sites where metastatic disease is found for many malignancies. Some lesions are discovered due to symptoms such as pneumonia, cough, hemoptysis or pain, but most are asymptomatic and are found on routine staging or surveillance imaging (1). A pulmonary metastasis is typically a well-circumscribed nodule, found in the periphery of the lung in two-thirds of cases (2). In contrast to screening for lung cancer, computed tomography (CT) scans performed on patients with a history of a previous cancer do not have a high false-positive rate (3). A new lesion that is larger than 1 cm very likely represents a malignant process if the clinical situation does not suggest infection.
Although many malignancies can metastasize to the lungs, the most common cancers for which pulmonary metastasectomy are considered and performed are epithelial cancers, sarcoma, melanoma and germ cell tumors. The epithelial malignancies for which pulmonary metastasectomy have been reported include gastrointestinal cancers, breast cancers, urothelial cancers, gynecological cancers, head and neck cancers, and thymic cancers. In current practice, pulmonary metastases are most commonly resected in patients with sarcoma and colorectal cancer (4).
Evidence supporting pulmonary metastasectomy
Randomized trials showing that pulmonary metastasectomy improves survival compared to non-resection management have not been performed (4). At present, pulmonary metastasectomy is offered to patients based on the observation that long-term survival can be seen after resection, while long-term survival with systemic therapy alone as treatment for patients with pulmonary metastases appears extremely unlikely (2). The data that supports pulmonary metastasectomy consists of registry data and non-controlled retrospective studies. These studies typically show good survival after pulmonary metastasectomy but have selection bias as an inherent limitation, in that the patients included in these studies by definition were considered potentially resectable and therefore likely had a limited number of metastases. These patients are therefore likely to have a better prognosis than other stage IV patients who have more widespread disease, and may have experienced prolonged survival even if pulmonary metastasectomy had not been performed (5,6).
Despite the lack of randomized data, many studies have documented reasonable survival after pulmonary metastasectomy. In an analysis from the International Registry of Lung Metastases which included 5,206 patients from 18 institutions in North America and Europe who underwent pulmonary metastasectomy from 1991 to 1995, complete resection was achieved in 4,572 (88%) patients (7). The actuarial survival for patients who underwent complete metastasectomy in this cohort was 36% at five years, 26% at ten years and 22% at 15 years. A single institution study of 490 patients who underwent complete metastasectomy at the European Institute of Oncology in Milan, Italy, for a wide distribution of primary cancers from 1998-2008 also showed a very reasonable actuarial five-year survival of 46% (8). Another multi-institution retrospective review of 378 patients who underwent pulmonary resection for colorectal cancer metastases with curative intent from 1998 to 2007, an era of modern chemotherapy, showed a 3-year overall survival of 78% (9). The 5-year survival in a series of 97 patients who underwent pulmonary resection for metastatic sarcoma was 50% (10).
Factors that are associated with improved survival after resection of pulmonary metastases have also been documented. In the analysis of the 5,206 patients in the International Registry of Lung Metastases, survival after pulmonary metastasectomy was best with smaller numbers of pulmonary metastases and longer intervals between diagnosis of the primary and the metastatic diseases (7). Completeness of resection, histology and disease-free interval greater than 36 months all predicted improved survival in the analysis of 490 patients from the European Institute of Oncology in Milan (8). In this cohort, prognosis was best for patients with germ cell tumors, followed by those with epithelial tumors, while patients with sarcoma and melanoma had the worst prognosis. In the analysis of 378 colorectal cancer patients, age younger than 65 years, female gender, a disease-free interval between primary and metastatic disease less than one year, and more than three metastases were all predictors of recurrence (9).
A randomized trial investigating colorectal metastasectomy is currently being performed (4). Until the results of that trial are reported, care will continue to be driven by the data from retrospective series. The decision to proceed with surgical resection of pulmonary metastases should be a multidisciplinary one, made jointly by the thoracic surgeon and the medical oncologist (1). Given that the benefits of resection in this setting have not been definitively established, avoiding both short-term and long-term morbidity for these patients who already have a poor prognosis is critical.
Criteria and goals for pulmonary metastasectomy
Several criteria establishing whether or not pulmonary metastasectomy is reasonable have been developed (1,2). First, the primary site of disease has to be either controlled or appear controllable. In addition, complete resection of pulmonary metastatic disease has to be feasible and anticipated to be tolerated by the patient. Finally, alternative therapies that are better than resection must not be available (2).
In order to achieve complete resection of pulmonary metastatic disease, surgeons often must plan for the resection of multiple and possibly bilateral lesions. Given that a new lesion that is larger than 1 cm on CT scan is very likely to represent a malignant process in a patient with a history of previous cancer if the clinical situation does not suggest infection, surgeons must plan to find and resect all suspicious lesions at the time of metastasectomy (3). The need to plan for the resection of multiple lesions, and the need to consider that a patient may require re-resection in the future if other metachronous lesions occur make the surgical management of metastatic lesions different from the surgical management of primary lung cancer. In addition, surgical management of primary lung cancer generally requires an anatomic resection for both staging purposes and to minimize the chance for local recurrence. In contrast, surgical management of a metastatic lesion only requires complete resection of each lesion with negative margins (11). When performing pulmonary metastasectomy, surgeons therefore must completely resect all lesions with negative margins while minimizing resection of functional lung tissue as much as possible, to ensure that both current and future lesions can be resected while leaving patients with adequate pulmonary function. Ultimately, the volume of disease, the location of the lesions, and the performance status of the patient guide the surgical approach (2).
Segmentectomy for pulmonary metastasectomy
Pulmonary metastasectomy must achieve resection of all lesions both identified on imaging before surgery and found intra-operatively, while preserving as much normal pulmonary parenchyma as possible (1). In contrast to primary lung cancer as described above, an anatomic pulmonary resection for metastatic disease does not improve survival compared to wedge resection (11). Because most pulmonary metastases are located in the lung periphery, resection most often requires wedge resection of the lung parenchyma. An anatomic resection is therefore indicated only when wedge resection would not achieve complete resection (2). More extensive surgical procedures such as lobectomy and pneumonectomy are sometimes technically necessary to allow complete resection of centrally located metastases. These more extensive resections may be appropriately indicated and offer some patients the best chance for long-term survival, but must be considered carefully as patients can subsequently develop metastases in the remaining lung, which could be unresectable depending on the patients’ previous resections.
Segmentectomy should be the first resection option carefully considered for all lesions that cannot be removed via wedge resection. As discussed above, pulmonary metastasectomies must accomplish the dual goals of achieving complete resection while preserving as much functional lung tissue as possible. Patients that undergo attempted complete resection of metastatic disease have been shown to have a significant loss of lung function. In 117 patients who underwent a variety of resections, the mean loss at three months after resection of percent-predicted FEV1 and percent-predicted DLCO from preoperative values was 10.8% and 9.7% respectively (12). Factors that predicted worse lung function were post-resection chemotherapy and bilateral procedures. Segmentectomy is associated with significant preservation of pulmonary function compared with lobectomy, and should be considered and explored for all lesions that do not absolutely technically require a lobectomy due to their central location (13,14).
Minimizing the amount of lung resected during metastasectomy is also important for preserving adequate functional lung tissue, as this allows the patient to undergo additional future resections if they develop metachronous lesions for which repeat metastasectomy is indicated. In the International Registry of Lung Metastases report, 20% of 5,206 patients underwent repeat resections; 5% of patients underwent three or more procedures overall (7). In addition, minimizing the extent of resection also likely improves perioperative outcomes. In the International Registry of Lung Metastases report, the operative mortality was 0.6% for sublobar resections, 1.2% for lobectomies and bilobectomies and 3.6% for pneumonectomies (7). The lack of definitive evidence proving a survival benefit to resection and the patients’ overall poor prognosis in general makes it even more critical to minimize its morbidity and subsequent impact on pulmonary function.
In general, pulmonary segmentectomies can be performed safely with acceptable morbidity and mortality. The 30-day mortality was 1.1% and the overall morbidity was 34.9% in one series of 785 anatomic segmentectomy patients, 41 of whom had a metastatic lesion resected (15). The major morbidity rate was 9.3%. Of 41 patients who had a segmentectomy for a metastatic lesion, 2 (4.9%) developed a locoregional recurrence. Resection of metastatic disease was the indication for surgery in 30 patients in another series of 77 segmentectomy patients (16). The mortality in this series was 2.6% (2 patients) and the morbidity was 32.5%. The most common complications were atrial arrhythmia (10 patients, 13%), pulmonary complications (9 patients, 12%) and prolonged air leak (7 patients, 9%). In these series, the performance of all common segmentectomies was reported including superior segmentectomy, basilar segmentectomy, lingulectomy and lingular-sparing upper lobectomy. In addition, segmental resections of the individual segments of the right upper and right middle lobe were also reported. Figure 1 shows some examples of central pulmonary metastases that were resected via segmentectomy.
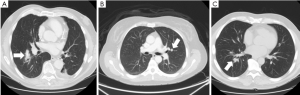
Anatomic segmentectomies are generally uncommonly used in the treatment of pulmonary metastases, accounting for between 3% and 23% of all resections in several relatively large series (7-9,17-19). Table 1 summarizes the use of segmentectomy in these series. The use of segmentectomy appears to be increasing over time, which may reflect increasing recognition of the importance of preserving pulmonary parenchyma for this disease process. Surgeons should consider segmentectomy for all cases where wedge resection is not feasible. Surgeons or centers that do not perform segmentectomy should consider referral to a center that does, to ensure that patients receive optimal care when undergoing pulmonary metastasectomy.
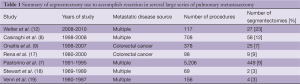
Full table
Use of minimally invasive approach
An area that is somewhat controversial is whether a minimally invasive technique with video-assisted thoracoscopic surgery (VATS) is appropriate for the resection of pulmonary metastases. Because manual lung palpation is limited with VATS, the identification of pulmonary nodules by VATS relies heavily on the preoperative CT scan and on the ability to visualize lesions in the periphery of the lung. However, pre-resection imaging with CT scans often underestimates the number of pulmonary nodules present (1,20). Metastases that are not detected on CT scan but are found when the lung is explored are noted in 16-46% of patients (3,21-24). Thoracoscopic resection of all lesions seen on CT scan with subsequent open exploration has also revealed missed metastases in 29-56% of patients (25,26). Although improvements with CT scans over time may decrease the number of missed nodules, many surgeons feel that using a thoracotomy so that the lung parenchyma can be fully palpated is essential and a VATS approach without palpation is suboptimal (3). In fact, an investigation of approach for pulmonary metastasectomy in the European Society of Thoracic Surgery (ESTS) practice patterns showed that 65 percent of surgeons thought palpation was necessary for adequate metastasectomy (27).
However, the data supporting the need to perform manual lung palpation via thoracotomy rather than reliance on imaging to guide resection is considered to be weak (3). Although multiple well-designed non-randomized studies have consistently shown that nodules are missed without palpation, studies have not shown that missing and not resecting these tiny nodules impacts survival. Several studies have not shown that a thoracotomy approach to pulmonary metastasectomy improves survival compared to VATS, although these studies are all somewhat limited by small sample sizes (28-30). A recent review of current data, which was noted to be limited to non-randomized retrospective studies that did not fully adjust for potential confounding factors, found no difference in survival between thoracoscopic and thoracotomy approaches (31). Thoracoscopic resection of metastases was associated with improved short-term outcomes in two studies, including shorter hospital stays, shorter chest drainage duration and fewer perioperative complications (28,29). Therefore, although the use of minimally invasive techniques limits manual palpation and therefore potential resection of small lesions not identified by pre-resection imaging, an approach of relying on imaging to guide resection via VATS is considered reasonable if careful follow-up is planned so that repeat resection of newly discovered nodules can be performed (3).
A VATS approach should be considered if segmentectomy for a metastasis is planned. Using minimally invasive techniques with thoracoscopy to perform segmentectomy has less short-term morbidity than thoracotomy. VATS segmentectomy has been shown to be a safe procedure that is associated with fewer complications and a reduced hospital stay when compared with an open segmentectomy (16,32). The VATS approach can be used for all potential segmental resections (15,16). The rates of conversion from VATS to open segmentectomy have been reported as 0-6.4%, with the most common reasons for conversion cited as inadequate exposure, hilar fibrosis and bleeding (15,16). The 30-day mortality in a series of 785 segmentectomies, of which a VATS approach was used for 468 patients, was 1.1% (15). There were no peri-operative mortalities in two smaller series of VATS segmentectomies (16,32). Table 2 summarizes several reports on the use of VATS to perform segmentectomies.
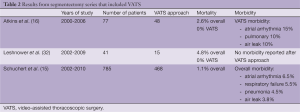
Full table
Conclusions
Pulmonary metastasectomy has a well-accepted role for certain primary cancers, in particular colorectal cancer and sarcoma, although this practice has not been proven by randomized trials to be more effective than non-operative management. However, patients have been observed to experience good long-term survival after resection of lung metastases, while long-term survival with systemic therapy alone as treatment for patients with pulmonary metastases is considered to be very unlikely. Because removal of all metastatic lesions has been consistently shown to be of great prognostic significance, surgeons must strive to remove as little lung tissue as possible while still achieving complete resection of each lesion. In this way, the patient will be able to tolerate resection of not only all synchronous disease but also possibly repeat resection if metachronous lesions develop. Segmentectomy has generally been infrequently utilized for pulmonary metastasectomy, but should be the first resection consideration if wedge resection technically cannot be performed for a lesion due to size or location. Avoiding lobectomy or even a more significant resection will allow a patient better preservation of pulmonary function, and likely allow them to tolerate resection of more lesions if necessary. Although the use of minimally invasive techniques limits manual palpation and therefore potential resection of small lesions not identified by pre-resection imaging, the current literature does not suggest that these procedures should be done via thoracotomy. Using VATS to perform segmentectomy is associated with less perioperative morbidity. However, careful follow-up surveillance imaging should be planned when manual palpation is not performed so that repeat resection of any new disease that appears can be considered.
Acknowledgements
Dr. Berry has received support from the National Institute of Health (NIH) funded Cardiothoracic Surgical Trials Network.
Disclosure: The author declares no conflict of interest.
References
- Rusch VW. Pulmonary metastasectomy. Current indications. Chest 1995;107:322S-331S. [PubMed]
- Ripley RT, Downey RJ. Pulmonary metastasectomy. J Surg Oncol 2014;109:42-6. [PubMed]
- Detterbeck FC, Grodzki T, Gleeson F, et al. Imaging requirements in the practice of pulmonary metastasectomy. J Thorac Oncol 2010;5:S134-9. [PubMed]
- Treasure T, Milosevic M, Fiorentino F, et al. Pulmonary metastasectomy: what is the practice and where is the evidence for effectiveness? Thorax 2014. [Epub ahead of print]. [PubMed]
- Treasure T, Fiorentino F, Scarci M, et al. Pulmonary metastasectomy for sarcoma: a systematic review of reported outcomes in the context of Thames Cancer Registry data. BMJ Open 2012;2:e001736. [PubMed]
- Aberg T, Malmberg KA, Nilsson B, et al. The effect of metastasectomy: fact or fiction? Ann Thorac Surg 1980;30:378-84. [PubMed]
- Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. The International Registry of Lung Metastases. J Thorac Cardiovasc Surg 1997;113:37-49. [PubMed]
- Casiraghi M, De Pas T, Maisonneuve P, et al. A 10-year single-center experience on 708 lung metastasectomies: the evidence of the “international registry of lung metastases”. J Thorac Oncol 2011;6:1373-8. [PubMed]
- Onaitis MW, Petersen RP, Haney JC, et al. Prognostic factors for recurrence after pulmonary resection of colorectal cancer metastases. Ann Thorac Surg 2009;87:1684-8. [PubMed]
- Kim S, Ott HC, Wright CD, et al. Pulmonary resection of metastatic sarcoma: prognostic factors associated with improved outcomes. Ann Thorac Surg 2011;92:1780-6; discussion 1786-7. [PubMed]
- Lo Faso F, Solaini L, Lembo R, et al. Thoracoscopic lung metastasectomies: a 10-year, single-center experience. Surg Endosc 2013;27:1938-44. [PubMed]
- Welter S, Cheufou D, Ketscher C, et al. Risk factors for impaired lung function after pulmonary metastasectomy: a prospective observational study of 117 cases. Eur J Cardiothorac Surg 2012;42:e22-7. [PubMed]
- Keenan RJ, Landreneau RJ, Maley RH Jr, et al. Segmental resection spares pulmonary function in patients with stage I lung cancer. Ann Thorac Surg 2004;78:228-33; discussion 228-33. [PubMed]
- Harada H, Okada M, Sakamoto T, et al. Functional advantage after radical segmentectomy versus lobectomy for lung cancer. Ann Thorac Surg 2005;80:2041-5. [PubMed]
- Schuchert MJ, Abbas G, Awais O, et al. Anatomic segmentectomy for the solitary pulmonary nodule and early-stage lung cancer. Ann Thorac Surg 2012;93:1780-5; discussion 1786-7. [PubMed]
- Atkins BZ, Harpole DH Jr, Mangum JH, et al. Pulmonary segmentectomy by thoracotomy or thoracoscopy: reduced hospital length of stay with a minimally-invasive approach. Ann Thorac Surg 2007;84:1107-12; discussion 1112-3. [PubMed]
- Rena O, Casadio C, Viano F, et al. Pulmonary resection for metastases from colorectal cancer: factors influencing prognosis. Twenty-year experience. Eur J Cardiothorac Surg 2002;21:906-12. [PubMed]
- Stewart JR, Carey JA, Merrill WH, et al. Twenty years’ experience with pulmonary metastasectomy. Am Surg 1992;58:100-3. [PubMed]
- Venn GE, Sarin S, Goldstraw P. Survival following pulmonary metastasectomy. Eur J Cardiothorac Surg 1989;3:105-9; discussion 110. [PubMed]
- McCormack PM, Ginsberg KB, Bains MS, et al. Accuracy of lung imaging in metastases with implications for the role of thoracoscopy. Ann Thorac Surg 1993;56:863-5; discussion 865-6. [PubMed]
- Margaritora S, Porziella V, D’Andrilli A, et al. Pulmonary metastases: can accurate radiological evaluation avoid thoracotomic approach? Eur J Cardiothorac Surg 2002;21:1111-4. [PubMed]
- Parsons AM, Ennis EK, Yankaskas BC, et al. Helical computed tomography inaccuracy in the detection of pulmonary metastases: can it be improved? Ann Thorac Surg 2007;84:1830-6. [PubMed]
- Cerfolio RJ, Bryant AS, McCarty TP, et al. A prospective study to determine the incidence of non-imaged malignant pulmonary nodules in patients who undergo metastasectomy by thoracotomy with lung palpation. Ann Thorac Surg 2011;91:1696-700; discussion 1700-1. [PubMed]
- Ellis MC, Hessman CJ, Weerasinghe R, et al. Comparison of pulmonary nodule detection rates between preoperative CT imaging and intraoperative lung palpation. Am J Surg 2011;201:619-22. [PubMed]
- Mutsaerts EL, Zoetmulder FA, Meijer S, et al. Outcome of thoracoscopic pulmonary metastasectomy evaluated by confirmatory thoracotomy. Ann Thorac Surg 2001;72:230-3. [PubMed]
- McCormack PM, Bains MS, Begg CB, et al. Role of video-assisted thoracic surgery in the treatment of pulmonary metastases: results of a prospective trial. Ann Thorac Surg 1996;62:213-6; discussion 216-7. [PubMed]
- Internullo E, Cassivi SD, Van Raemdonck D, et al. Pulmonary metastasectomy: a survey of current practice amongst members of the European Society of Thoracic Surgeons. J Thorac Oncol 2008;3:1257-66. [PubMed]
- Mutsaerts EL, Zoetmulder FA, Meijer S, et al. Long term survival of thoracoscopic metastasectomy vs metastasectomy by thoracotomy in patients with a solitary pulmonary lesion. Eur J Surg Oncol 2002;28:864-8. [PubMed]
- Gossot D, Radu C, Girard P. Resection of pulmonary metastases from sarcoma: can some patients benefit from a less invasive approach? Ann Thorac Surg 2009;87:238-43. [PubMed]
- Nakas A, Klimatsidas MN, Entwisle J, et al. Video-assisted versus open pulmonary metastasectomy: the surgeon’s finger or the radiologist’s eye? Eur J Cardiothorac Surg 2009;36:469-74. [PubMed]
- Greenwood A, West D. Is a thoracotomy rather than thoracoscopic resection associated with improved survival after pulmonary metastasectomy? Interact Cardiovasc Thorac Surg 2013;17:720-4. [PubMed]
- Leshnower BG, Miller DL, Fernandez FG, et al. Video-assisted thoracoscopic surgery segmentectomy: a safe and effective procedure. Ann Thorac Surg 2010;89:1571-6. [PubMed]





