The application of capnography to differentiate peri-chest tube air leak from parenchymal leak following pulmonary surgery
Introduction
Air leaks represent a common complication following pulmonary resection (1,2). In most individuals, these will resolve in the early postoperative period but persistent air leaks are demonstrated to result in prolonged hospital stays, increased costs and risk of infection (3-5). Attention has been given to intraoperative measures to limit the incidence of air leaks such as sealants and staple line buttresses; however, their efficacy remains unclear (6).
The postoperative management of chest tubes therefore remains an essential component of patient care. Whilst it is acceptable to manage persistent air leaks on an outpatient basis with a Heimlich valve or similar compact drainage device, this can be uncomfortable for patients and is demonstrated to lower patient satisfaction (7). The goal of the surgeon is therefore to optimize the inpatient assessment of air leaks and ensure safe and early removal of chest tubes.
Previously, there was little to guide the surgeon in the timing of chest tube removal other than clinical experience. Recent developments have led to the use of digital air leak devices, which give a quantitative measure of the air leak size (8). However, these devices are costly, and often clinical decisions remain subjectively based on the observation of bubbles in the chest drain (9). A major limitation of this approach is the inability to determine whether the bubbles are representative of a pulmonary air leak or of air drawn into the pleural cavity via an incomplete seal of the tissues around the chest tube. The latter occurs due to the negative intra-thoracic pressures generated during respiration, and is particularly evident in thin patients in whom tissue closure often fails to achieve an adequate seal during prolonged drainage.
In this article, we describe a simple method to determine the nature of chest drain bubbling which, in our practice, has optimized the postoperative management of pulmonary resection patients.
Methods
Following observation of chest drain bubbling, clinical examination is performed, with attention given to auscultation and percussion of the chest and the inspection of any surgical wounds. If necessary, a plain chest radiograph is performed to confirm the presence or absence of a significant pneumothorax. Whilst a pneumothorax may be evident, these routine interventions cannot confirm an associated pulmonary air leak.
The technique described here relies on the detection of raised CO2 levels in the chest drainage system to confirm a pulmonary air leak. If chest drain bubbling is a result of air entering the pleural space via the chest tube wound, then the levels of CO2 in the chest drainage system are expected to be normal [similar to the atmosphere].
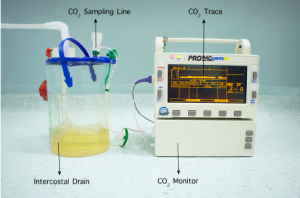
A Propaq® Encore Vital Signs Monitor (WelchAllyn® NY, USA) is attached to the chest drain via a standard CO2 sampling line. Alternative handheld vital signs monitors used in the intensive care unit settings may also be suitable for this task. The elbow connector of the sampling line is attached to the exhaust of the chest drain system (Figure 1).
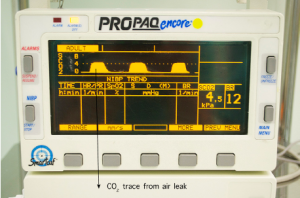
The sidestream CO2 option is selected, which will display the measured CO2 levels as both a waveform and a numeric value. The patient is then asked to take some controlled, deep breaths whilst the resultant waveform is observed. In the event of a pulmonary air leak, the monitor will display a characteristic CO2 waveform (Figure 2). Conversely, it is assumed that chest tube bubbling is a result of air drawn through the chest tube wound in the absence of a CO2 waveform.
Comment
This simple technique can prevent chest tubes being left in unnecessarily and has greatly improved our management of chest drainage systems in postoperative patients. Using this technique has also reduced the number of patients discharged home with compact chest drainage systems. Whilst described here as an aid to managing drains following pulmonary resection, the technique is equally applicable to other surgical procedures including bullectomy and lung biopsy. In summary, we have found the technique described here to be safe, cost-effective and reliable at confirming the presence or absence of a pulmonary air leak following pulmonary resection.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Rice TW, Kirby TJ. Prolonged air leak. Chest Surg Clin North Am 1992;2:803-11.
- Rice TW, Okereke IC, Blackstone EH. Persistent air-leak following pulmonary resection. Chest Surg Clin N Am 2002;12:529-39. [PubMed]
- Bardell T, Petsikas D. What keeps postpulmonary resection patients in hospital? Can Respir J 2003;10:86-9. [PubMed]
- Varela G, Jiménez MF, Novoa N, et al. Estimating hospital costs attributable to prolonged air leak in pulmonary lobectomy. Eur J Cardiothorac Surg 2005;27:329-33. [PubMed]
- Brunelli A, Xiume F, Al Refai M, et al. Air leaks after lobectomy increase the risk of empyema but not of cardiopulmonary complications: a case-matched analysis. Chest 2006;130:1150-6. [PubMed]
- Singhal S, Shrager JB. Should buttresses and sealants be used to manage pulmonary parenchymal air leaks? J Thorac Cardiovasc Surg 2010;140:1220-5. [PubMed]
- Cerfolio RJ, Bass CS, Pask AH, et al. Predictors and treatment of persistent air leaks. Ann Thorac Surg 2002;73:1727-30; discussion 1730-1.
- Dernevik L, Belboul A, Rådberg G. Initial experience with the world’s first digital drainage system. The benefits of recording air leaks with graphic representation. Eur J Cardiothorac Surg 2007;31:209-13. [PubMed]
- Cerfolio RJ. Recent advances in the treatment of air leaks. Curr Opin Pulm Med 2005;11:319-23. [PubMed]