Spinal cord protection and related complications in endovascular management of B dissection: LSA revascularization and CSF drainage
Introduction
The endovascular repair of thoracic aorta (TEVAR) has significantly decreased the overall incidence of neurologic complications when compared with open surgery. Nevertheless, the risk of paraplegia remains an important concern, with rates ranging from 2% up to 8% (1). Risk factors for spinal cord ischemia following TEVAR include prior abdominal aortic aneurysm (AAA) repair, prolonged hypotension, severe atherosclerosis of the thoracic aorta, occlusion of the left subclavian artery (LSA) or hypogastric arteries, and more extensive coverage of the thoracic aorta by the graft (1).
Different strategies have been developed over time to protect the spinal cord from ischemic insult during thoracic aortic repair (2) (see Table 1). LSA revascularization and cerebral spinal fluid (CSF) drainage are the two more invasive preventive maneuvers applied in TEVAR for treating type B dissection which may be associated with relevant pitfalls.
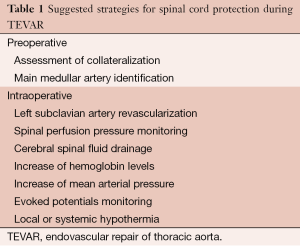
Full table
Pitfalls and safeguards
LSA revascularization pitfalls
Since the effective contribution of the LSA to spinal cord vascularization is difficult to estimate due to anatomical variability, its revascularization may be not always necessary and should be performed selectively. Most authors agree on absolute indications to LSA revascularization in patients presenting with specific clinical situations, including left internal mammary artery-coronary bypass, dominant left vertebral artery, isolated left cerebral hemisphere, and functioning left upper extremity artery-venous dialysis fistula or bypasses. A selective revascularization approach has also been suggested based on ischemic risk stratification, such as in the presence of extensive aortic coverage, prior aortic surgery, or an occluded hypogastric artery (3). LSA revascularization may be associated with several complications, which are summarized in Table 2 (4).

Full table
LSA revascularization safeguards
Recommendations to avoid such complications focus on two aspects: a justified indication, and a careful and skillful surgical technique to avoid injuries to adjacent structures when a left subclavian-carotid transposition or bypass grafting is performed. Because the surgical outcome is strongly related to volume and experience, highly experienced surgeons should be in charge of these surgical preventive procedures.
CSF drainage pitfalls
Spinal fluid pressure (SFP) normally approximates central venous pressure, and spinal cord perfusion pressure is the difference between mean arterial pressure and SFP. Thoracic endograft deployment produces an acute rise in both central venous pressure and SFP. This increase in SFP decreases spinal cord blood flow. For this reason, CSF drainage is recommended in selected TEVAR cases which have a high risk of spinal cord ischemia (Table 3), as part of a multimodality approach for the prevention of neurological complications.
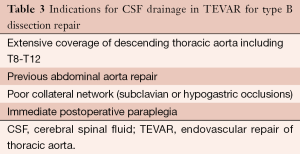
Full table
Changes in spinal fluid volume and pressure affect intracranial mechanics and can produce deleterious clinical effects. When spinal fluid is removed, SFP drops, producing intracranial hypotension. This can cause acute intracranial (intraparenchymal, subdural, or subarachnoid) bleeding due to the enlargement and rupture of venous sinuses or cortical veins and caudal brain displacement.
In addition, besides the particular complications associated with lumbar puncture or neuraxial anesthesia (i.e., bleeding puncture, headache, infection), in patients necessitating a CSF monitoring and draining catheter, brain bleeding complications (Table 4) are the most serious events which can occur (5). There are several risk factors for brain bleeding related to CSF drainage (Table 5).

Full table
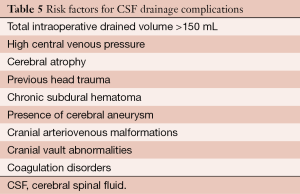
Full table
CSF drainage safeguards
It is very important to identify any risk factor listed in Table 5. The presence of any coagulation disorders as well as antiplatelet or anticoagulant therapy must be taken into account. To avoid bleeding complications, antiplatelet treatment should be stopped at least 5 days before surgery and the catheter should be removed at least six hours after last low molecular weight heparin dosage.
Continuous and controlled CSF monitoring and drainage should be used. No more than 10 mL/hour should be drained and SFP should be maintained at 10-12 mmHg, avoiding any inadvertent catheter obstruction.
As a general rule, the main goal is to reach a control SFP with the minimum volume of spinal fluid drainage in order to minimize intracranial bleeding complications. During the postoperative phase, the catheter must be kept in place for 24-72 hours, carefully maintaining spinal cord perfusion pressure above 70 mmHg. If bloody liquid is observed through the catheter, drainage must be stopped; coagulation parameters should be checked and corrected if necessary. Additionally, spinal and head computed tomography or magnetic resonance scans should be performed in patients with bloody spinal fluid, as well as in all patients with abnormal neurologic signs.
The spinal catheter can be removed after a period of 12 hours, with stopped drainage in order to check for any neuro-deficit and correct the coagulation parameters. Besides CSF drainage, important adjuvant perioperative preventive measures which should be taken include maintaining mean blood pressure above 80 mmHg and hemoglobin level above 10 g/dL.
To avoid complications related to management of the CSF catheter, a dedicated and automated CSF pressure and drain monitoring system, the LiquoGuard® (Möller Medical GmbH & Co KG, Fulda, Germany) has been recently proposed (6). This has a transducer and a console which measures CSF pressure, connected to a peristaltic tube pump which drains CSF continuously, according to pre-established parameters and limits for CSF pressure (hourly drainage speed, maximum and minimum CSF pressure, maximum amount to be drained per hour). LiquoGuard® facilitates detection of catheter leakage and helps avoid catheter occlusions by ensuring a continuous and controlled CSF flow. Moreover, the LiquoGuard® sensor unit is easily taped and adjusted to the lumbar catheter level, by using a specially designed fixation device, for maximal patient comfort and mobility. This system permits a continuous pressure-controlled CSF flow with a high level of patient and nurse compliance, with on-line and off line recording capabilities.
Comments
Both LSA revascularization and CSF drainage are recommended as the most prominent maneuvers to prevent paraplegia, the most dramatic complication in thoracic aorta repairs. However, both need to be applied in selected patients who may benefit the most from them and who justify the implicit and potential serious complications. To minimize such pitfalls, both techniques should be carefully performed. Concerning CSF drainage, beyond the technique itself, anesthesiologists and nurses must pay special attention on CSF pressure and drained volume, mean arterial pressure and coagulation monitoring. Recent specific tools, such as LiquoGuard®, make CSF pressure and monitoring easier.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Amabile P, Grisoli D, Giorgi R, et al. Incidence and determinants of spinal cord ischaemia in stent-graft repair of the thoracic aorta. Eur J Vasc Endovasc Surg 2008;35:455-61. [PubMed]
- Sinha AC, Cheung AT. Spinal cord protection and thoracic aortic surgery. Curr Opin Anaesthesiol 2010;23:95-102. [PubMed]
- Matsumura JS, Lee WA, Mitchell RS, et al. The Society for Vascular Surgery Practice Guidelines: management of the left subclavian artery with thoracic endovascular aortic repair. J Vasc Surg 2009;50:1155-8. [PubMed]
- Maldonado TS, Dexter D, Rockman CB, et al. Left subclavian artery coverage during thoracic endovascular aortic aneurysm repair does not mandate revascularization. J Vasc Surg 2013;57:116-24. [PubMed]
- Wynn MM, Mell MW, Tefera G, et al. Complications of spinal fluid drainage in thoracoabdominal aortic aneurysm repair: a report of 486 patients treated from 1987 to 2008. J Vasc Surg 2009;49:29-34; discussion 34-5. [PubMed]
- Riambau V, Matute P. Automatic monitoring of spinal fluid drainage: Safer protection for spinal cord ischemia. In: Greenhalgh R. eds. Vascular and Endovascular Consensus update. Biba Medical, London, 2011;61-6.





