Endovascular management of chronic post-dissection aneurysms
Introduction
Although rare, aortic dissection is one of the most feared diseases of the aorta, associated with high mortality and morbidity especially in the acute setting (1-4). According to the Stanford classification system, 60% of dissections are classified as type A and the remaining 40% as type B (2). Surgery is the treatment of choice for patients with type A dissections, in order to treat or prevent common lethal complications such as aortic rupture, stroke, visceral ischemia, cardiac tamponade and circulatory failure. The treatment rationale for type B dissections is more controversial. Type B dissections are usually classified as acute (≤14 days) or chronic (>14 days) according to the onset of symptoms. Medical treatment was long considered the treatment of choice for acute uncomplicated type B dissections, but newer insights seem to favor endovascular repair in a larger proportion of uncomplicated acute type B dissections (5). Treatment in the acute phase is clearly advocated for dissections complicated by rupture, abdominal end-organ ischemia, lower limb ischemia, persistent pain, and refractory hypertension (4,6). Endovascular treatment is an established method in the treatment of acute complicated type B dissections, with several studies suggesting that endovascular management provides better survival than open surgery (7-10). However, the role of endovascular repair in the treatment of chronic dissection remains, less clear.
Natural history of aortic dissection
A proportion of patients surviving an acute aortic dissection will develop chronic post-dissection aneurysms that will need further treatment.
Mid- and long-term survival estimates of patients successfully treated for acute type A dissection are improving, with recent reported 1-year survival estimates of 90-97.7% and 5-year estimates of 76-88.2% (11,12). Patent false lumen in descending aortic segments after surgical treatment of type A dissection is common, with reported median aortic growth rates varying from 1 to 3.7 mm/year (13,14). In a recent study by Evangelista et al., 9% of patients with treated type A dissections required further therapy during follow-up due to descending aortic dilation (15).
In-hospital outcomes are generally acceptable in patients with uncomplicated acute type B dissections, 90% of whom survive to hospital discharge after receiving effective hypertensive therapy, with 30-day mortality as low as 3.3% (16). A proportion of patients ranging from 20-40% will develop a post-dissection aneurysm, requiring interventional management to prevent rupture in 18% of cases (6,17). Factors associated with secondary aneurysm formation include initial false lumen diameter and patency of the false lumen. According to Song et al., initial diameter of the false lumen at the proximal descending aorta of ≥2 cm predicts aneurysm formation with a sensitivity of 100% and a specificity of 76% (18). Patency of the false lumen and especially partial false lumen thrombosis have been studied in phantom models and are considered to be independent predictors of aneurysm formation and late adverse outcomes (4,19). According to Blount et al., aortic diameter growth after type B dissection occurs at a mean rate of 7.1 mm/year (20).
Mean aortic growth rates after acute type A and B dissection indicate that post-dissection aneurysm formation is a long degenerative process occurring over a period of several years. This process often involves the thoracoabdominal aorta and is accompanied by extensive aortic remodeling leading to fibrotic stiffness of the dissection flap and resulting in a secondary aneurysm that presents with diverse technical challenges for treatment.
Treatment of post-dissection aneurysms
The indication to treat a post-dissection aneurysm is almost exclusively related to aortic dilation. All other indications for treatment, such as late malperfusion, pleural effusion and persistent pain are rare and could be included in a category of subacute symptoms. The treatment goal in chronic dissection is therefore no different to treating non-dissected aneurysms: the aneurysm needs to be excluded completely.
Open repair
Open surgical techniques for thoracoabdominal aneurysms are technically demanding and are associated with high mortality and morbidity rates. Current literature focusing exclusively on open surgical repair of secondary post-dissection aneurysms is sparse. Studies from high-volume centers including both post-dissecting and atherosclerotic aneurysms report a 30-day mortality rate of 5-8% with a paraplegia risk approaching 6-8% (21-24). Renal complications develop in 17-25% of patients, with up to 15% requiring hemodialysis. Whilst these results are acceptable, taking the nature of the pathology into consideration, they primarily reflect the extensive experience of the highest volume centers. Analyses of volume-related outcomes over a wider range of hospitals reveal the “real world” picture, with overall mortality reaching 22.3%, and postoperative complication rates exceeding 55% (25).
Due to its major invasiveness, and the associated mortality, open surgery in the thoracoabdominal aortic segment can only be justified in patients in reasonably good condition. It is clear that post-dissection thoracoabdominal aneurysms do represent an additional technical challenge for the surgeon, which could result in even higher rates of mortality and morbidity.
Standard thoracic endovascular aneurysm repair (TEVAR)
In acute and sub-acute dissections, coverage of the proximal intimal tear seems to promote false lumen thrombosis and aortic remodeling (26). This treatment option has been applied in TEVAR for selected post-dissection aneurysms, but patient selection and indication is not well defined, and results are heterogeneous (27).
Several studies have reported positive results after endovascular repair of chronic type B dissections with early mortality rates of 0-7.5% (28-31). A recent systematic review by Thrumurthy et al. demonstrates an overall 30-day mortality of 3.2% (27). Reported neurologic complication rates range from 0% to 9% (3,29). Standard TEVAR seems to excel over open surgery with regard to early mortality and morbidity.
Mid-term results of standard TEVAR remain, however, controversial. Individual survival rates vary from 59.1% to 100% in studies with a median follow-up of 24 months (27). According to Kang et al., postoperative aortic remodeling (i.e., decrease in aortic diameter) in patients with chronic dissection seems to be limited to the stent-grafted segment of the aorta, not influencing the distal untreated segments (32). A recent report by Mani et al. demonstrated that the rate of aortic remodeling is dependent on the extent of the aortic dissection and the presence of false lumen thrombosis. Although total false lumen thrombosis was achieved in 83% of patients with the dissection confined to the thoracic aorta, this was the case for only 23% of patients with dissection extending into the abdomen. Patients with less than total false lumen thrombosis were more likely to experience aortic expansion over time, which was the strongest predictor of mid-term mortality (33). According to Andacheh et al., TEVAR is a potential treatment option for patients with chronic type B dissection. However, many patients with extension of thoracic dissection into the infrarenal aorta demonstrated continued aortic dilation and an increased need for secondary measures for persistent distal perfusion (34).
In our view, standard TEVAR is a viable approach only for post-dissection aneurysms limited to the thoracic aorta. Complete thrombosis of the entire false lumen after standard thoracic endografting is uncommon in patients with extensive chronic dissections, mainly due to increasing fibrotic stiffness of the aortic lamellae that limits the remodeling potential of the dissected aorta. The purpose of treatment in this case is a complete exclusion of the aneurysmal degeneration with sealing of proximal as well as distal entry tears. To achieve this, a more extensive endovascular approach is required.
Fenestrated and branched TEVAR (F/Br-TEVAR)
In post-dissection aneurysms involving the thoracoabdominal aorta, complete aneurysm exclusion by endovascular means requires utilization of fenestrated/branched stent-grafts.
F/Br-TEVAR for thoracoabdominal aortic aneurysms (TAAAs) was initially limited to a few pioneering centers, but is now entering its second decade and has widespread use in large-volume endovascular centers throughout the world. The favorable results and durability of fenestrated and branched grafts for atherosclerotic TAAAs have been demonstrated in several reports (35-39). Despite the obvious advantages of F/Br-TEVAR as a less invasive treatment, operative risks still remain. Mortality has been reported to be within 5-20%, with the larger series reporting mortality rates less than 10%. Spinal cord ischemia varies among published series, and seems to have a more benign profile compared to open surgery (i.e., later onset, transient). Renal failure remains a problem and can occur due to intra-procedural technical errors, late occlusions of renal artery stent-grafts, and multiple contrast-enhanced CT scans. F/Br-TEVAR for TAAA is a complex procedure but has shown encouraging results and has the potential to become the treatment of choice for non-dissected TAAAs.
Experience with F/Br-TEVAR in the treatment of post dissection aneurysms has been very limited until recently, mainly due to the additional technical difficulties when treating this etiological subgroup of TAAAs. Specific literature on F/Br-TEVAR in patients with chronic type B dissection is still sparse (16,40,41).
The most specific feature in this pathology is the narrow true lumen of the aorta. This needs to be taken into account when deciding whether to use fenestrations or branches, and makes it difficult to decide on the orientation of both fenestrations and branches. Fenestrations require less true lumen space, but must be catheterized from below and require more accurate planning with regard to orientation. In addition, repositioning a fenestrated graft to catheterize the target vessels can be tedious in a post-dissection aneurysm. Branched grafts are easier to plan but the branches require extra room, both for correct deployment and catheterization. This room usually is lacking in post-dissection aneurysms, but extra room can be created by deployment of a tube graft first with a distal landing zone a few centimeters above the visceral branches of the aorta. This usually helps to expand the true lumen somewhat.
A second technical challenge relates to visceral branches originating from the false lumen. This applies particularly to either of the renal arteries. In order to correctly catheterize target vessels originating from the false lumen, the thick chronic dissection flap has to be perforated, or a re-entry tear found. Several methods can be used to address the issue: wires with tips that can be stiffened, the back of a wire, a wire with support of a guiding sheath, or even a needle to perforate the dissection flap as used in transjugular intrahepatic portosystemic shunt procedures.
Nuremberg experience
Our initial experience with fenestrated/branched grafts to treat secondary aneurysms has shown favorable early results (40). During the period January 2010 to November 2013, 17 patients (13 males, mean age 65±7.8 years) with chronic thoracoabdominal aneurysmal degeneration following acute dissection were treated in our department with the use of fenestrated/branched stent-grafts (Table 1). The procedure was carried out under general anesthesia in all patients. An adjunct trans-axillary approach was used in 13 (76.5%) patients. Median operative time was 280 min (range, 130-420 min) and median estimated blood loss (EBL) 440 mL (range, 100-1,000 mL). Median fluoroscopy time was 63 min (range, 24-112 min) and mean iodinated contrast volume used 213±103 mL. Spinal drainage was applied in 15 (88.3%) patients.
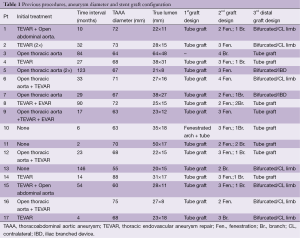
Full table
Technical success was achieved in all cases (100%). There were 2 (11.8%) deaths in the early post-operative period. One died due to multiple organ failure on the first postoperative day. Computed tomography angiography (CTA) showed no signs of target vessel occlusion, but demonstrated renal and intestinal micro-infarction. The second patient suffered a deterioration of cardiac function, ultimately resulting in cardiac failure and death, following a technically successful procedure. One (5.9%) patient with impaired pre-operative renal function required permanent dialysis at five months. Two (11.8%) patients suffered spinal cord ischemia with temporary paraparesis. No cases of paraplegia occurred.
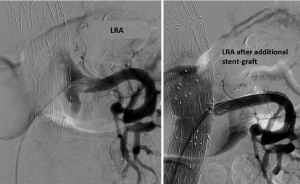
Median follow-up was 12 months (range, 4-28 months). In three cases, the length of the bridging covered stent proved too short after remodeling, resulting in a type Ib endoleak from a side branch. These were all successfully treated with a longer bridging stent-grafts (Figure 1). Type II endoleaks were diagnosed in five cases with three of them resolving spontaneously. No aneurysm-related deaths were observed during follow-up. Complete false lumen thrombosis at 6-month follow-up was present in 8/11 (72.7%) patients.
Conclusions
The treatment goal in chronic post-dissection aneurysms is complete exclusion. Endovascular repair offers a viable alternative to open surgery. Standard TEVAR is feasible for post-dissection aneurysms limited to the thoracic segment of the aorta. Thoracoabdominal post-dissection aneurysms require a more extensive endovascular approach with fenestrated/branched stent-grafts. In view of the promising early results, fenestrated/branched stent-grafts may in the future become the first treatment option for post-dissection aneurysms.
Acknowledgements
Eric Verhoeven is consultant for Cook, W.L. Gore & Associates, Siemens, and Atrium.
Disclosure: The authors declare no conflict of interest.
References
- Svensson LG, Kouchoukos NT, Miller DC, et al. Expert consensus document on the treatment of descending thoracic aortic disease using endovascular stent-grafts. Ann Thorac Surg 2008;85:S1-41. [PubMed]
- Daily PO, Trueblood HW, Stinson EB, et al. Management of acute aortic dissections. Ann Thorac Surg 1970;10:237-47. [PubMed]
- Zipfel B, Czerny M, Funovics M, et al. Endovascular treatment of patients with types A and B thoracic aortic dissection using Relay thoracic stent-grafts: results from the RESTORE Patient Registry. J Endovasc Ther 2011;18:131-43. [PubMed]
- Tsai TT, Trimarchi S, Nienaber CA. Acute aortic dissection: perspectives from the International Registry of Acute Aortic Dissection (IRAD). Eur J Vasc Endovasc Surg 2009;37:149-59. [PubMed]
- Hughes GC, Andersen ND, McCann RL. Management of acute type B aortic dissection. J Thorac Cardiovasc Surg 2013;145:S202-7. [PubMed]
- Fanelli F, Dake MD. Standard of practice for the endovascular treatment of thoracic aortic aneurysms and type B dissections. Cardiovasc Intervent Radiol 2009;32:849-60. [PubMed]
- Khoynezhad A, Donayre CE, Omari BO, et al. Midterm results of endovascular treatment of complicated acute type B aortic dissection. J Thorac Cardiovasc Surg 2009;138:625-31. [PubMed]
- Feezor RJ, Martin TD, Hess PJ Jr, et al. Early outcomes after endovascular management of acute, complicated type B aortic dissection. J Vasc Surg 2009;49:561-6; discussion 566-7. [PubMed]
- Verhoye JP, Miller DC, Sze D, et al. Complicated acute type B aortic dissection: midterm results of emergency endovascular stent-grafting. J Thorac Cardiovasc Surg 2008;136:424-30. [PubMed]
- Fattori R, Tsai TT, Myrmel T, et al. Complicated acute type B dissection: is surgery still the best option?: a report from the International Registry of Acute Aortic Dissection. JACC Cardiovasc Interv 2008;1:395-402. [PubMed]
- Fattouch K, Sampognaro R, Navarra E, et al. Long-term results after repair of type a acute aortic dissection according to false lumen patency. Ann Thorac Surg 2009;88:1244-50. [PubMed]
- Zierer A, Voeller RK, Hill KE, et al. Aortic enlargement and late reoperation after repair of acute type A aortic dissection. Ann Thorac Surg 2007;84:479-86; discussion 486-7. [PubMed]
- Fattori R, Bacchi-Reggiani L, Bertaccini P, et al. Evolution of aortic dissection after surgical repair. Am J Cardiol 2000;86:868-72. [PubMed]
- Halstead JC, Meier M, Etz C, et al. The fate of the distal aorta after repair of acute type A aortic dissection. J Thorac Cardiovasc Surg 2007;133:127-35. [PubMed]
- Evangelista A, Salas A, Ribera A, et al. Long-term outcome of aortic dissection with patent false lumen: predictive role of entry tear size and location. Circulation 2012;125:3133-41. [PubMed]
- Trimarchi S, Righini P, Grassi V, et al. Do branched and fenestrated devices have a role in chronic type B aortic dissection? J Cardiovasc Surg (Torino) 2011;52:529-38. [PubMed]
- Schor JS, Yerlioglu ME, Galla JD, et al. Selective management of acute type B aortic dissection: long-term follow-up. Ann Thorac Surg 1996;61:1339-41. [PubMed]
- Song JM, Kim SD, Kim JH, et al. Long-term predictors of descending aorta aneurysmal change in patients with aortic dissection. J Am Coll Cardiol 2007;50:799-804. [PubMed]
- Sueyoshi E, Sakamoto I, Hayashi K, et al. Growth rate of aortic diameter in patients with type B aortic dissection during the chronic phase. Circulation 2004;110:II256-61. [PubMed]
- Blount KJ, Hagspiel KD. Aortic diameter, true lumen, and false lumen growth rates in chronic type B aortic dissection. AJR Am J Roentgenol 2009;192:W222-9. [PubMed]
- Coselli JS, Bozinovski J, LeMaire SA. Open surgical repair of 2286 thoracoabdominal aortic aneurysms. Ann Thorac Surg 2007;83:S862-4; discussion S890-2.
- Coselli JS, LeMaire SA, Conklin LD, et al. Left heart bypass during descending thoracic aortic aneurysm repair does not reduce the incidence of paraplegia. Ann Thorac Surg 2004;77:1298-303; discussion 1303. [PubMed]
- Misfeld M, Sievers HH, Hadlak M, et al. Rate of paraplegia and mortality in elective descending and thoracoabdominal aortic repair in the modern surgical era. Thorac Cardiovasc Surg 2008;56:342-7. [PubMed]
- Wong DR, Parenti JL, Green SY, et al. Open repair of thoracoabdominal aortic aneurysm in the modern surgical era: contemporary outcomes in 509 patients. J Am Coll Surg 2011;212:569-79; discussion 579-81. [PubMed]
- Cowan JA Jr, Dimick JB, Henke PK, et al. Surgical treatment of intact thoracoabdominal aortic aneurysms in the United States: hospital and surgeon volume-related outcomes. J Vasc Surg 2003;37:1169-74. [PubMed]
- Kusagawa H, Shimono T, Ishida M, et al. Changes in false lumen after transluminal stent-graft placement in aortic dissections: six years’ experience. Circulation 2005;111:2951-7. [PubMed]
- Thrumurthy SG, Karthikesalingam A, Patterson BO, et al. A systematic review of mid-term outcomes of thoracic endovascular repair (TEVAR) of chronic type B aortic dissection. Eur J Vasc Endovasc Surg 2011;42:632-47. [PubMed]
- Sayer D, Bratby M, Brooks M, et al. Aortic morphology following endovascular repair of acute and chronic type B aortic dissection: implications for management. Eur J Vasc Endovasc Surg 2008;36:522-9. [PubMed]
- Parsa CJ, Williams JB, Bhattacharya SD, et al. Midterm results with thoracic endovascular aortic repair for chronic type B aortic dissection with associated aneurysm. J Thorac Cardiovasc Surg 2011;141:322-7. [PubMed]
- Manning BJ, Dias N, Ohrlander T, et al. Endovascular treatment for chronic type B dissection: limitations of short stent-grafts revealed at midterm follow-up. J Endovasc Ther 2009;16:590-7. [PubMed]
- Virtue Registry Investigators. The VIRTUE Registry of type B thoracic dissections--study design and early results. Eur J Vasc Endovasc Surg 2011;41:159-66. [PubMed]
- Kang WC, Greenberg RK, Mastracci TM, et al. Endovascular repair of complicated chronic distal aortic dissections: intermediate outcomes and complications. J Thorac Cardiovasc Surg 2011;142:1074-83. [PubMed]
- Mani K, Clough RE, Lyons OT, et al. Predictors of outcome after endovascular repair for chronic type B dissection. Eur J Vasc Endovasc Surg 2012;43:386-91. [PubMed]
- Andacheh ID, Donayre C, Othman F, et al. Patient outcomes and thoracic aortic volume and morphologic changes following thoracic endovascular aortic repair in patients with complicated chronic type B aortic dissection. J Vasc Surg 2012;56:644-50; discussion 650. [PubMed]
- Verhoeven EL, Tielliu IF, Ferreira M, et al. Thoraco-abdominal aortic aneurysm branched repair. J Cardiovasc Surg (Torino) 2010;51:149-55. [PubMed]
- Guillou M, Bianchini A, Sobocinski J, et al. Endovascular treatment of thoracoabdominal aortic aneurysms. J Vasc Surg 2012;56:65-73. [PubMed]
- Anderson JL, Adam DJ, Berce M, et al. Repair of thoracoabdominal aortic aneurysms with fenestrated and branched endovascular stent grafts. J Vasc Surg 2005;42:600-7. [PubMed]
- Verhoeven EL, Tielliu IF, Bos WT, et al. Present and future of branched stent grafts in thoraco-abdominal aortic aneurysm repair: a single-centre experience. Eur J Vasc Endovasc Surg 2009;38:155-61. [PubMed]
- Haulon S, D’Elia P, O’Brien N, et al. Endovascular repair of thoracoabdominal aortic aneurysms. Eur J Vasc Endovasc Surg 2010;39:171-8. [PubMed]
- Verhoeven EL, Paraskevas KI, Oikonomou K, et al. Fenestrated and branched stent-grafts to treat post-dissection chronic aortic aneurysms after initial treatment in the acute setting. J Endovasc Ther 2012;19:343-9. [PubMed]
- Kitagawa A, Greenberg RK, Eagleton MJ, et al. Fenestrated and branched endovascular aortic repair for chronic type B aortic dissection with thoracoabdominal aneurysms. J Vasc Surg 2013;58:625-34. [PubMed]





