Predictors of false lumen thrombosis in type B aortic dissection treated with TEVAR
Introduction
Type B aortic dissection (TBAD) is a potentially life threatening cardiovascular event, which is associated with significant morbidity and mortality (1,2). Uncomplicated TBAD patients have traditionally been managed medically, while complications like aortic rupture, mesenteric ischemia, limb ischemia, uncontrolled hypertension and refractory pain mandate direct surgical intervention (3,4). Thoracic endovascular aortic repair (TEVAR) has replaced conventional open surgery as it is associated with lower morbidity and mortality, and it is currently the first-line treatment option in many acute and chronic dissections (5-7). During these procedures, the proximal tear is covered by a stent graft to re-establish normal antegrade blood flow in the true lumen (TL). By excluding perfusion of the false lumen (FL), thrombosis is induced and regression of the thrombus will eventually result in aortic remodeling (8-10).
Recent studies have shown that FL thrombosis (FLT) and aortic remodeling are associated with better long-term outcomes and is considered as one of the primary outcome measures for TEVAR (8-10). A patent FL after TEVAR, comparable to those treated medically, can lead to aneurysmal dilatation and potential rupture. The clinical significance of FLT and aortic remodeling has recently been emphasized, as it is associated with better five-year survival (11). Despite these promising results, the primary mortality and the number of reinterventions remains high in TEVAR patients, which can be mainly attributed to periprocedural complications (4,7). By enhancing the understanding regarding which subset of patients are most likely to benefit from such procedures, a better assessment can be made not only to decide on which patient we should intervene, but also to determine the type and extent of the procedure. In this study, we sought to identify pre- and postoperative morphologic characteristics that are associated with FLT, in order to predict outcomes in patients who undergo TEVAR for TBAD.
Methods
All patients treated at our institution between December 2005 and July 2012 for a TBAD with TEVAR were considered eligible for this study. Patients were included based on the availability of preoperative, postoperative and a CT angiogram (CTA) at 1-year follow-up. Type A aortic dissections, intramural hematomas, penetrating aortic ulcers and previous descending aortic replacement were considered exclusion criteria. Demographic information, operative details and follow-up data were retrospectively obtained. Institutional review board (IRB) approval was obtained (IRB # 12020).
Data collection
CT scan data were transferred to a workstation with Aquarius iNtuition (TeraRecon, San Mateo, Calif, USA) for semiautomatic volume measurement with a center lumen line method. Automated segmentation, which determines boundaries around voxels with a similar intensity, was used and manually adjusted in order to select the TL and total aortic lumen. Subsequently, the TL and overall aortic volume were calculated automatically. All volumes were analyzed for both the descending aorta, from the left subclavian artery (LSA) to the celiac artery, and abdominal aorta, from the celiac trunk to the aortic bifurcation. FL volume was obtained by subtracting the TL volume from the total aortic volume on both the abdominal and thoracic levels. Changes in volumes were calculated by the following formula: [(volume at 1-year follow-up – volume postoperative)/volume postoperative] ×100. All diameter measurements were performed perpendicular to the center lumen line.
Pre-operative and post-operative morphologic predictors
The configuration of the dissection was classified as straight or spiral, with a spiral dissection defined as a dissection where the middle of the FL changed position at least 90 degrees, compared to the TL, at any level between the LSA and the aortic bifurcation. The LSA, celiac artery, superior mesenteric artery, left and right renal artery, inferior mesenteric artery and both iliac arteries were investigated with the use of transverse, coronal, sagittal and multiplanar reconstructions, whether side branches of the aorta were involved on the baseline CTA scan. Side branch involvement was considered when the FL partially or completely vascularized one or more of these branches. The location of the FL was classified as present in the inner or outer curve of the aortic arch. The aortic diameter at the level of the proximal and distal landing zone, the maximum diameter of the descending aorta, length of the dissection and distance from the beginning of the dissection to the LSA were measured.
On the postoperative scan, we quantified the following measurements and predictors: total treatment length, distance from proximal stentgraft to the LSA, distance from distal landing zone and the celiac artery, the presence of patent entry tears, FL patency, retrograde perfusion of the FL, and the presence of endoleaks.
Study end-points
Full FLT, defined as the absence of contrast in the FL, was objectified on CTA obtained after 1-year of follow-up.
Analysis
Descriptive statistics are reported as mean ± standard deviation (SD) or median and interquartile range (IQR). Statistical analyses were performed using the χ2-test for categorical variables and t-test for continuous variables. Predictors of FLT where explored using univariate logistic regression analysis. Variables with a P value<0.1 were included for multivariate logistic regression to investigate independent predictors. Statistical analysis was conducted with SPSS software version 20.0 (SPSS Inc, Chicago, III, USA).
Results
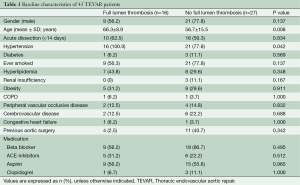
Of 132 patients that received TEVAR for TBAD, 43 patients (mean age 60.3±14.2; 30 male) met our inclusion criteria, of which 16 (37%) developed FLT after 1 year of follow-up. Demographics showed that FLT-patients tended to be older (66.3±8.9 vs. 56.7±15.5; P=0.008) and had more frequently a history of hypertension (100% vs. 77.8%; P=0.042) while no differences were observed among acute TBAD (62.5% vs. 59.3%; P=0.834. Table 1).
Full table
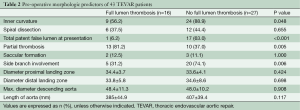
Preoperative images showed that FLT patients presented more often with a dissection situated in the outer curvature of the aorta compared with non-FLT patients (56.2% vs. 88.9%; P=0.048; Table 2). FL status differed between groups, with 6.2% of the FLT-patient had a patent FL at presentation compared to 63% of the non-FLT patients (P<0.001). Side-branch vessels were involved in 74.1% of the non-FLT patients compared to 31.2% of the FLT group (P value=0.006). Landing zone diameter, length of dissection, and total length of aorta were not predictive of FLT.
Full table
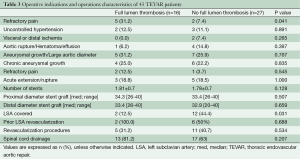
Operative indications and characteristics are summarized in Table 3. Aneurysmal dilatation or an aortic diameter >5.5 cm was most often the indication for TEVAR in both the acute and chronic setting. Only refractory pain was more frequently observed in FLT-patients. Coverage of the LSA was necessary in 12 patients (12.5% in FLT vs. 44.4% in non-FLT; P=0.031), with eight patients undergoing prior LSA revascularization. Other revascularization procedures included a stent in a visceral or iliac artery in eight patients and a fenestration between the true and FL in one patient.
Full table
Postoperative imaging showed that two-thirds of the FLT patients did not have a patent entry tear after the initial procedure, while almost 90% of the non-FT patients had one or more patent entry tears (P<0.001; Table 4). Complete lumen thrombosis was present in 62.5% of the FT patients at the post-operative scan. Endoleaks were more often present in non-FT patients, with two third of the non-FT patients presenting with retrograde filling of the FL. In addition, FT-patients tended to have an increased treatment length (202±67.0 mm vs. 186.9±54.6 mm; P=0.101), which probably also resulted in the distal portion of stent graft being deployed closer to the celiac artery (63.4±52.3 mm vs. 108.3±59.1 mm; P=0.014).
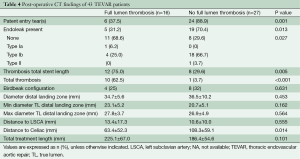
Full table
Outcome multivariate logistic regression model
Multivariate logistic regression showed that side-branch involvement [odds ratio (OR), 0.03; 95% confidence interval (CI), 0.00-0.92; P=0.045] and a total patent FL at presentation (OR, 0.01; 95% CI, 0.00-0.58; P=0.027; Table 5) were associated with decreased full FLT.
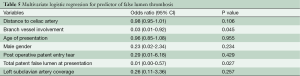
Full table
Outcome volumetric data
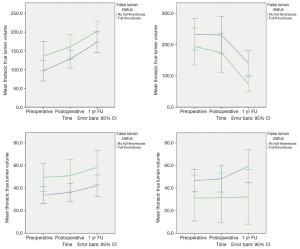
Volumetric data analyses showed that both subsets of patients had an increase of the thoracic TL volume and decrease in FL volume after stent graft placement and during follow-up (Figure 1). Changes in TL volume were comparable for both groups, but we found a more significant reduction of the thoracic FL in FLT patients compared with non-FLT (–52.3% vs. –32.4%; P=0.043; Tables 6,7) There was a tendency towards less volume increase in the abdominal segment. (–5.0 ± 37.5 vs. 21.8 ± 44.3; P=0.052)

Full table

Full table
Patient outcome
Mean follow-up was 2.7±1.6 years, during which 21 patients required reintervention. In the non-FLT group, two patients required open arch repair, one ascending and total arch replacement for retrograde type A aorta dissection and four patients required open repair for thoracoabdominal aorta aneurysms. Other reinterventions included proximal extension for type I endoleak, coil embolization for type II endoleaks and EVAR for abdominal aneurysm (Table 8).

Full table
Discussion
Acute TBAD is a cardiovascular catastrophe associated with high morbidity and mortality (1,2). According to current guidelines, medical treatment should be offered to those presenting with uncomplicated aortic dissection, while TEVAR should be reserved for those patients presenting with malperfusion or aortic rupture (3,4). The recent long-term outcomes of the INSTEAD-xl trial have shown that TEVAR in uncomplicated acute TBAD is associated with better aortic remodeling and improved long-term aorta-specific survival compared to medical therapy alone (11). However, the drawbacks of TEVAR are not isolated to early mortality, but also include the number of reinterventions, which is relative high in TBAD compared to TEVAR in thoracic aortic aneurysms (5,7). FLT portends a better outcome in these patients, as persistent pressurization of the FL is associated with aortic related events like aortic dilatation and rupture. Via coverage of the entry tear and directing blood-flow to the TL, stent graft placement can induce FLT, promoting remodeling of the aorta and preventing late expansion and malperfusion (8,9). Our analyses showed that patients with a patent FL at presentation and branch vessel involvement are less likely to develop FLT and potential have a worse outcome.
Previous reports showed that FLT can be obtained in 40-90% of TBAD patients treated with TEVAR, which is relative high compared to our results of 32% (8-10,12,13). Our analyses showed that FLT at 1 year was predominantly obtained in patients with (partial) FLT at presentation. These findings suggest that patients with high flow in the FL, as a result from sufficient in and outflow, are less likely to benefit from TEVAR. This observation is supported by the fact that 90% of the non-FLT patients still had a patent entry tear after TEVAR. This confirmed previous findings of Kusagawa et al., who showed that remaining intimal tears in the thoracic aorta hamper shrinkage of the thoracic FL (14). However, outflow or retrograde perfusion of the FL can also be constituted by perfusion through branch vessels, which was confirmed by our multivariate analyses. Our findings showed that branch vessel involvement indicates that the presence of re-entries is predictive of reduced FLT and aortic remodeling, thus warranting additional endovascular interventions or even potential conversion to open surgical repair. Therefore, patients where all vessels originate from the TL might present a favorable subgroup.
The extent of distal coverage remains controversial since the primary focus of TEVAR in TBAD focusses on coverage of the proximal entry tear. The drawback of this approach is that it overlooks additional problems encountered more distally, such as additional re-entry tears, branch vessel involvement, or a collapsed TL. While we routinely place a stent graft over the proximal entry tear and reserve deployment of additional grafts to those presenting with a major endoleak or TL collapse, the use of two-component devices might address these issues in a better fashion (15). With this technique, standardized TEVAR is supplemented with placement of uncovered stent over the entire length of the dissection in order to appose the dissected segment to the aortic wall thereby stabilizing the lamella, while preserving blood-flow to the visceral vessels and spinal cord. Indications for this technique are currently dynamic malperfusion and distal TL collapse. Our findings suggest that this technique might be extended to those patients with an entry-tear near the branch vessels or involvement of one the branch vessels. Some groups demonstrated that the petticoat technique stabilized the abdominal segment and was beneficial maintaining the overall aortic volumes, although they did not observe remodeling of the abdominal aorta (15-17). Nevertheless, before such techniques are implemented in practice in the United States, further studies are mandatory.
We observed favorable TL expansion and FL reduction for both group over time. In our analysis, we found significant more reduction of the thoracic FL in FT patients compared with non-FT (–52.3% vs. –32.4%; P=0.043), while there also was a tendency to significant reduction in the abdominal aorta for FT-patients as continuous perfusion of the FL in the abdominal aorta can lead to a significant increase in abdominal aortic diameter. In the non-FT group, four patients required open surgical repair for type IV aneurysms during follow-up, showing the clinical importance of FLT over the entire length of the aorta. Previous reports showed that smaller preoperative TL volumes and smaller maximum descending aorta diameter were independent predictors of postoperative FT, which could not be confirmed by our analyses (18). However, the post-operative reduction of the FL was significantly more distinct in FT-patients, suggesting that if there is a tendency to remodeling, this will become apparent directly after surgery.
There are several limitations in the present study, including its retrospective design, single-center site and a small number of patients, which limits the power of the study. The short follow-up time might have influenced the results, as the benefits of TEVAR will likely become more prominent after 2 years. In addition, static CTA does not provide the correct visualization of this dynamic disease process. Future studies using 4D PC-MRI, which can both visualize and quantify flow characteristics in relationship to aortic expansion, will allow us to better understand this dynamic disease in the future (19).
Conclusions
This study was performed in order to better define those patients that would most likely benefit from TEVAR following TBAD. Our study showed that patients with a fully patent FL at presentation and branch vessel involvement are less likely to develop full FLT and thus benefit from standard TEVAR. These patients may require a more extensive procedure, with more aortic coverage and more aggressive stent grafting of end organ vessels, such as the mesenteric and renal, to prevent long-term complications.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA 2000;283:897-903. [PubMed]
- Tsai TT, Fattori R, Trimarchi S, et al. Long-term survival in patients presenting with type B acute aortic dissection: insights from the International Registry of Acute Aortic Dissection. Circulation 2006;114:2226-31. [PubMed]
- Hiratzka LF, Bakris GL, Beckman JA, et al. 22010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the diagnosis and management of patients with thoracic aortic disease. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. J Am Coll Cardiol 2010;55:e27-e129. [PubMed]
- Svensson LG, Kouchoukos NT, Miller DC, et al. Expert consensus document on the treatment of descending thoracic aortic disease using endovascular stent-grafts. Ann Thorac Surg 2008;85:S1-41. [PubMed]
- Bavaria JE, Appoo JJ, Makaroun MS, et al. Endovascular stent grafting versus open surgical repair of descending thoracic aortic aneurysms in low-risk patients: a multicenter comparative trial. J Thorac Cardiovasc Surg 2007;133:369-77. [PubMed]
- Beregi JP, Haulon S, Otal P, et al. Endovascular treatment of acute complications associated with aortic dissection: midterm results from a multicenter study. J Endovasc Ther 2003;10:486-93. [PubMed]
- Eggebrecht H, Nienaber CA, Neuhäuser M, et al. Endovascular stent-graft placement in aortic dissection: a meta-analysis. Eur Heart J 2006;27:489-98. [PubMed]
- Conrad MF, Crawford RS, Kwolek CJ, et al. Aortic remodeling after endovascular repair of acute complicated type B aortic dissection. J Vasc Surg 2009;50:510-7. [PubMed]
- Rodriguez JA, Olsen DM, Lucas L, et al. Aortic remodeling after endografting of thoracoabdominal aortic dissection. J Vasc Surg 2008;47:1188-94. [PubMed]
- Sayer D, Bratby M, Brooks M, et al. Aortic morphology following endovascular repair of acute and chronic type B aortic dissection: implications for management. Eur J Vasc Endovasc Surg 2008;36:522-9. [PubMed]
- Nienaber CA, Kische S, Rousseau H, et al. Endovascular repair of type B aortic dissection: long-term results of the randomized investigation of stent grafts in aortic dissection trial. Circ Cardiovasc Interv 2013;6:407-16. [PubMed]
- Eriksson MO, Steuer J, Wanhainen A, et al. Morphologic outcome after endovascular treatment of complicated type B aortic dissection. J Vasc Interv Radiol 2013;24:1826-33. [PubMed]
- Parsa CJ, Schroder JN, Daneshmand MA, et al. Midterm results for endovascular repair of complicated acute and chronic type B aortic dissection. Ann Thorac Surg 2010;89:97-102. [PubMed]
- Kusagawa H, Shimono T, Ishida M, et al. Changes in false lumen after transluminal stent-graft placement in aortic dissections: six years' experience. Circulation 2005;111:2951-7. [PubMed]
- Lombardi JV, Cambria RP, Nienaber CA, et al. Prospective multicenter clinical trial (STABLE) on the endovascular treatment of complicated type B aortic dissection using a composite device design. J Vasc Surg 2012;55:629-40.e2.
- Melissano G, Bertoglio L, Rinaldi E, et al. Volume changes in aortic true and false lumen after the "PETTICOAT" procedure for type B aortic dissection. J Vasc Surg 2012;55:641-51. [PubMed]
- Nienaber CA, Kische S, Zeller T, et al. Provisional extension to induce complete attachment after stent-graft placement in type B aortic dissection: the PETTICOAT concept. J Endovasc Ther 2006;13:738-46. [PubMed]
- Stanley GA, Murphy EH, Knowles M, et al. Volumetric analysis of type B aortic dissections treated with thoracic endovascular aortic repair. J Vasc Surg 2011;54:985-92. [PubMed]
- Clough R, Taylor P. Future imaging techniques in aortic pathologies and clinical implications. J Cardiovasc Surg (Torino) 2013;54:15-9. [PubMed]