Predicting aortic enlargement in type B aortic dissection
Perspectives
In the absence of complications such as visceral malperfusion, renal failure, periaortic hematoma or rupture, refractory pain and/or hypertension, patients with an uncomplicated acute type B aortic dissection (ABAD) are currently treated conservatively (1). Despite adequate antihypertensive treatment, delayed aortic dilatation will develop in 20-50% of patients with uncomplicated ABAD, which can lead to the catastrophic event of aortic dilatation and rupture (2-4). In light of this, some randomized controlled trials studied the importance of prophylactic thoracic endovascular aortic repair (TEVAR) in uncomplicated ABAD to prevent such complications. These studies failed to show TEVAR was beneficial in the short-term (5). Recently however, a more positive long-term outcome after TEVAR has been demonstrated (6). Despite this potential long-term benefit, TEVAR may also be associated with complications including aortic rupture, retrograde dissection, and stent graft-related complications such as stent graft migration and endoleaks. Therefore, a conservative approach is still advocated for most patients (7-9).
By identifying patients at risk for aortic enlargement at an early stage, a subset of ABAD may profit from stricter follow-up and even prophylactic intervention. In particular, a significant group of patients that develop widespread aneurysmal degeneration along the dissected segments during follow-up may lose the chance for endovascular treatment if not identified at an early stage. In this report, we have summarized the current literature regarding clinical and radiologic predictors of aortic growth in conservatively treated uncomplicated ABAD patients.
Clinical predictors
The age of ABAD patients appears to affect aortic expansion during follow-up. Patients younger than 60 years have shown to exhibit a significantly increased aortic growth rate, while a decreased growth rate has been associated with increasing age (10,11). This may be attributed to the fact that as the aortic wall becomes more rigid during life, due to degeneration and the presence of atherosclerotic factors, it becomes less prone to dilate. Moreover, aortic dissection in younger individuals identifies a cohort with a higher chance of genetic abnormalities that contribute to degradation of aortic elastic elements over time (12). Previous reports have shown that presence of connective tissue disorders, primarily Marfan syndrome, result in an increased risk of aortic enlargement and death during follow-up after ABAD (13,14).
The role of gender in favoring aortic growth in ABAD patients is not completely understood. Recent reports showed that men experienced a higher growth rate than women (11,15), while aortic enlargement was previously described to be higher in women (10). The conflicting nature of such observations remains unresolved. Furthermore, racial differences appear to exist. A report of the International Registry of Acute Aortic Dissection (IRAD) showed that Caucasian background was associated with increased risk of aortic enlargement, whilst patients of Asian background were associated with a decreased risk of aortic enlargement (15). These findings are supported by a significantly lower prevalence of abdominal aortic aneurysm in the Asian population compared with the Caucasian population (16,17).
During follow-up, patients with appropriate blood pressure control (systolic blood pressure <120 mmHg) and a tight control of heart rate (<60 beats/min) have shown improved outcome with less aortic events, including aortic expansion and rupture, compared with patients with a heart rate ≥60 beats/min and appropriate blood pressure control (18). These findings emphasize the importance of adequate heart rate and blood pressure control, not only in the acute phase but also during follow-up. Therefore, careful treatment and strict follow-up is required in all ABAD patients.
Beside β-blockers for control of heart rate and blood pressure, calcium channel antagonists may also be indicated for blood pressure control, because patients treated with calcium channel antagonists have been demonstrated to exhibit less aortic enlargement (15). In addition to these observational data, some clinical trials have established the beneficial effect of calcium channel blockers in hypertension control (19,20). The association of these antihypertensive drugs may be useful for treating ABAD in the long-term.
At admission, certain blood tests may also have predictive value. Kitada et al. showed that fibrinogen-fibrin degradation product (FDP) level >20 mg/mL at admission was associated with aortic enlargement, while other laboratory factors, such as thrombin-antithrombin III complex, D-dimer, platelet count and co-reactive protein were not associated with aortic enlargement (21). Long-term follow-up of these markers remains undefined, because the follow-up interval in this study was only 1 year. Although FDP has not been introduced as a routine screening tool, blood tests for such biomarkers, in association with other predictors, may be useful in identifying those ABAD patients at higher risk for aortic enlargement at an early stage. Future research should investigate whether additional biomarkers for thoracic aortic aneurysm may also be predictive for aortic enlargement in uncomplicated ABAD patients (22).
Radiologic predictors
A maximum aortic diameter of >40 mm in the acute phase was marked as a predictor of aortic growth in several studies, while a diameter of <40 mm was a negative predictor (12,23-26). Most studies define aortic enlargement as a maximum diameter of the dissected aorta of >60 mm or rapid enlargement of 10 mm/year, or both, given that such findings indicate surgical intervention. Higher aortic growth rates in these patients are associated with the fact that patients with a higher initial maximum diameter will generally reach the boundary of 60 mm earlier than patients with a smaller diameter (10,12,23). In addition to these findings, the IRAD data demonstrated that an initial diameter of <40 mm was also predictive for aortic expansion during follow-up (15). Thus, in addition to patients with a high initial aortic diameter, patients with a lower initial aortic diameter may also present with a significant aortic enlargement and therefore should be monitored more closely than previously assumed. As alluded to, a potential explanation would be a higher genetic influence in patients with aortic dissection at a small size in this particular group of patients. Nevertheless, a selection bias may have influenced the results: large dissected aortas were excluded for analysis because they generally more often undergo early intervention (15).
Focusing on the aortic diameter in ABAD patients, a distinction between the total aortic diameter and the false lumen (FL) diameter can be made. Song et al. found that a FL diameter of >22 mm in the upper thoracic descending aorta on the initial computed tomography imaging was an independent predictor for aortic enlargement during follow-up (27). The initial FL diameter correlated with the rate of aortic dilatation at the proximal descending aorta in patients who showed aortic growth during follow-up. Moreover, the ratio of FL diameter to total aortic diameter increased at the proximal and middle descending thoracic aorta in those patients (27). A large FL diameter reflects high pressure in the FL. Therefore it may play an essential role in aortic dilatation of the FL and the development of thoracic aortic aneurysm.
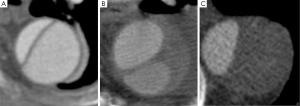
In addition, the configuration of the FL reflects the intraluminal pressure. An elliptical configuration of the true lumen (TL) in combination with a circular formation of the FL has been reported to be associated with increased aortic growth (Figure 1) (11). This radiologic configuration is the result of higher pressurization of the FL, which effects the shape of the TL, subjects the FL to higher radial forces, and potentially leads to aortic enlargement (11). Similarly, a circular TL configuration suggests a high pressure in the TL compared with the FL, and this radiologic sign was associated with a decreased growth rate (11).

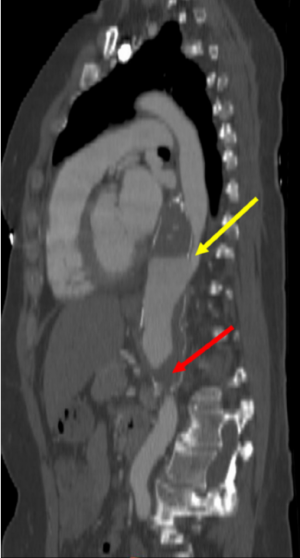
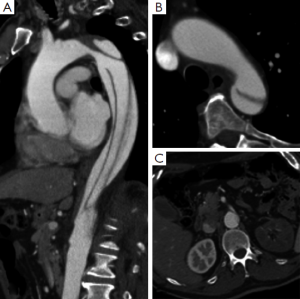
Several studies have identified that the presence of blood flow in the FL predicts aortic enlargement (10,12,24,25,28). A patent FL causes direct hemodynamic stress and structural weakening of the aortic wall, which might induce progressive growth of the affected aortic segment, while a completely thrombosed FL has been associated with less aortic enlargement and aortic wall remodeling (12,24,29). The importance of a partially thrombosed FL is even more controversial, since a previous IRAD report documented the association between partial FL thrombosis and lower survival at 3 years during follow-up in ABAD patients (14). Whilst other groups have not found a relationship between partially thrombosed FL and aortic enlargement, more recent studies have shown that partial lumen thrombosis is predictive for aortic enlargement (Figure 2) (11,29,30). In this setting, Sueyoshi and colleagues observed that patients with a saccular formation of the FL, defined as partial thrombosis in the distal portion of the FL with proximal filling of the FL, forming a blind sac, demonstrated a significantly higher growth rate compared with no sac formation (29). These findings were supported by another more recent study (Figure 3) (11). The aortic enlargement may be explained by a higher diastolic FL pressure due to occlusion of distal re-entry tears. In line with these findings, previous reports demonstrated that a patent primary entry site in the thorax was predictive for aortic enlargement compared with an undetected primary entry site (23).
Recently, Tolenaar and colleagues found that the number of entry tears at initial imaging was associated with aortic enlargement during follow-up (31). Patients with one entry tear at presentation show a higher growth rate than patients with more entry tears (31). The normal blood flow might change into turbulent flow due to the presence of only one patent entry tear and absence of a distal re-entry, with pressurization of the FL. This phenomenon causes higher stress of the weakened dissected aortic wall and subsequent aortic enlargement (32). Moreover, Evangelista and colleagues demonstrated that patients with a large primary entry tear (>10 mm) presented more often with dissection-related events and showed a higher growth rate than those with a smaller entry tear (<10 mm) (13). A larger tear size suggests that more blood enters the FL, leading to increased FL pressurization and consequent aortic enlargement.
These findings are supported by in vitro studies that demonstrate that a large tear size presents with lower velocity through the tears but more complex flows in the FL, while small tears resulted in higher tear velocities and a well-defined flow with lower FL pressure (33). Another in vitro study showed that systolic pressure in the FL decreases with smaller tear size, and similarly, higher FL pressure with larger tear size will increase wall shear stress and may cause aortic dilatation (34).
Multiple radiologic predictors, including patent FL, FL diameter >22 mm, elliptic TL combined with round FL, one entry tear, and entry tear size >10 mm, all seem inter-related due to pressurization of the FL, with subsequent aortic enlargement of the dissected segment. One study found that a FL located in the inner curvature of the aorta was associated with aortic enlargement. This was in concordance with another experience that showed that a primary entry tear in the inner curvature/concavity was associated with the development of complications (Figure 4) (11,35). Congruently with these findings, a FL located at the inner aortic curvature seems associated with a higher incidence of aortic complications in clinical practice, both in the acute and chronic setting. However, there is no consensus yet and future studies are required to corroborate these observations.
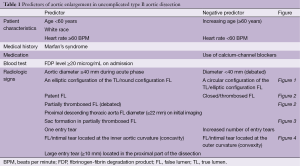
The different predictors for aortic enlargement in uncomplicated ABAD patients are summarized in Table 1. Combining these clinical and radiologic predictors may be essential to identify patients with uncomplicated ABAD at higher risk for aortic enlargement and rupture during follow-up.
Full table
Conclusions
Several predictors might be used to identify those ABAD patients at high risk for aortic enlargement during follow-up. Although conservative management remains indicated in all uncomplicated ABAD, particularly during the acute phase, these patients might benefit from closer radiologic surveillance or early endovascular intervention. This approach deserves even more consideration because a significant group of patients develops aneurysmal degeneration along the dissected segments during follow-up and may lose the chance for endovascular treatment if not identified at an early stage.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Tolenaar JL, van Bogerijen GH, Eagle KA, et al. Update in the management of aortic dissection. Curr Treat Options Cardiovasc Med 2013;15:200-13. [PubMed]
- Akin I, Kische S, Rehders TC, et al. Thoracic endovascular stent-graft therapy in aortic dissection. Curr Opin Cardiol 2010;25:552-9. [PubMed]
- Fattori R, Tsai TT, Myrmel T, et al. Complicated acute type B dissection: is surgery still the best option?: a report from the International Registry of Acute Aortic Dissection. JACC Cardiovasc Interv 2008;1:395-402. [PubMed]
- Umaña JP, Lai DT, Mitchell RS, et al. Is medical therapy still the optimal treatment strategy for patients with acute type B aortic dissections? J Thorac Cardiovasc Surg 2002;124:896-910. [PubMed]
- Fattori R, Montgomery D, Lovato L, et al. Survival after endovascular therapy in patients with type B aortic dissection: a report from the International Registry of Acute Aortic Dissection (IRAD). JACC Cardiovasc Interv 2013;6:876-82. [PubMed]
- Nienaber CA, Kische S, Rousseau H, et al. Endovascular repair of type B aortic dissection: long-term results of the randomized investigation of stent grafts in aortic dissection trial. Circ Cardiovasc Interv 2013;6:407-16. [PubMed]
- Brunkwall J, Lammer J, Verhoeven E, et al. ADSORB: a study on the efficacy of endovascular grafting in uncomplicated acute dissection of the descending aorta. Eur J Vasc Endovasc Surg 2012;44:31-6. [PubMed]
- Nienaber CA, Rousseau H, Eggebrecht H, et al. Randomized comparison of strategies for type B aortic dissection: the INvestigation of STEnt Grafts in Aortic Dissection (INSTEAD) trial. Circulation 2009;120:2519-28. [PubMed]
- Nienaber CA, Kische S, Akin I, et al. Strategies for subacute/chronic type B aortic dissection: the Investigation Of Stent Grafts in Patients with type B Aortic Dissection (INSTEAD) trial 1-year outcome. J Thorac Cardiovasc Surg 2010;140:S101-8; discussion S142-6.
- Sueyoshi E, Sakamoto I, Hayashi K, et al. Growth rate of aortic diameter in patients with type B aortic dissection during the chronic phase. Circulation 2004;110:II256-61. [PubMed]
- Tolenaar JL, van Keulen JW, Jonker FH, et al. Morphologic predictors of aortic dilatation in type B aortic dissection. J Vasc Surg 2013;58:1220-5. [PubMed]
- Marui A, Mochizuki T, Mitsui N, et al. Toward the best treatment for uncomplicated patients with type B acute aortic dissection: A consideration for sound surgical indication. Circulation 1999;100:II275-80. [PubMed]
- Evangelista A, Salas A, Ribera A, et al. Long-term outcome of aortic dissection with patent false lumen: predictive role of entry tear size and location. Circulation 2012;125:3133-41. [PubMed]
- Tsai TT, Evangelista A, Nienaber CA, et al. Partial thrombosis of the false lumen in patients with acute type B aortic dissection. N Engl J Med 2007;357:349-59. [PubMed]
- Jonker FH, Trimarchi S, Rampoldi V, et al. Aortic expansion after acute type B aortic dissection. Ann Thorac Surg 2012;94:1223-9. [PubMed]
- Salem MK, Rayt HS, Hussey G, et al. Should Asian men be included in abdominal aortic aneurysm screening programmes? Eur J Vasc Endovasc Surg 2009;38:748-9. [PubMed]
- Spark JI, Baker JL, Vowden P, et al. Epidemiology of abdominal aortic aneurysms in the Asian community. Br J Surg 2001;88:382-4. [PubMed]
- Kodama K, Nishigami K, Sakamoto T, et al. Tight heart rate control reduces secondary adverse events in patients with type B acute aortic dissection. Circulation 2008;118:S167-70. [PubMed]
- Jamerson KA, Bakris GL, Wun CC, et al. Rationale and design of the avoiding cardiovascular events through combination therapy in patients living with systolic hypertension (ACCOMPLISH) trial: the first randomized controlled trial to compare the clinical outcome effects of first-line combination therapies in hypertension. Am J Hypertens 2004;17:793-801. [PubMed]
- Redón J, Trenkwalder PR, Barrios V. Efficacy of combination therapy with angiotensin-converting enzyme inhibitor and calcium channel blocker in hypertension. Expert Opin Pharmacother 2013;14:155-64. [PubMed]
- Kitada S, Akutsu K, Tamori Y, et al. Usefulness of fibrinogen/fibrin degradation product to predict poor one-year outcome of medically treated patients with acute type B aortic dissection. Am J Cardiol 2008;101:1341-4. [PubMed]
- van Bogerijen GH, Tolenaar JL, Grassi V, et al. Biomarkers in TAA-the Holy Grail. Prog Cardiovasc Dis 2013;56:109-15. [PubMed]
- Kato M, Bai H, Sato K, et al. Determining surgical indications for acute type B dissection based on enlargement of aortic diameter during the chronic phase. Circulation 1995;92:II107-12. [PubMed]
- Kunishige H, Myojin K, Ishibashi Y, et al. Predictors of surgical indications for acute type B aortic dissection based on enlargement of aortic diameter during the chronic phase. Jpn J Thorac Cardiovasc Surg 2006;54:477-82. [PubMed]
- Onitsuka S, Akashi H, Tayama K, et al. Long-term outcome and prognostic predictors of medically treated acute type B aortic dissections. Ann Thorac Surg 2004;78:1268-73. [PubMed]
- Winnerkvist A, Lockowandt U, Rasmussen E, et al. A prospective study of medically treated acute type B aortic dissection. Eur J Vasc Endovasc Surg 2006;32:349-55. [PubMed]
- Song JM, Kim SD, Kim JH, et al. Long-term predictors of descending aorta aneurysmal change in patients with aortic dissection. J Am Coll Cardiol 2007;50:799-804. [PubMed]
- Akutsu K, Nejima J, Kiuchi K, et al. Effects of the patent false lumen on the long-term outcome of type B acute aortic dissection. Eur J Cardiothorac Surg 2004;26:359-66. [PubMed]
- Sueyoshi E, Sakamoto I, Uetani M. Growth rate of affected aorta in patients with type B partially closed aortic dissection. Ann Thorac Surg 2009;88:1251-7. [PubMed]
- Trimarchi S, Tolenaar JL, Jonker FH, et al. Importance of false lumen thrombosis in type B aortic dissection prognosis. J Thorac Cardiovasc Surg 2013;145:S208-12. [PubMed]
- Tolenaar JL, van Keulen JW, Trimarchi S, et al. Number of entry tears is associated with aortic growth in type B dissections. Ann Thorac Surg 2013;96:39-42. [PubMed]
- Cheng Z, Tan FP, Riga CV, et al. Analysis of flow patterns in a patient-specific aortic dissection model. J Biomech Eng 2010;132:051007. [PubMed]
- Rudenick PA, Bijnens BH, García-Dorado D, et al. An in vitro phantom study on the influence of tear size and configuration on the hemodynamics of the lumina in chronic type B aortic dissections. J Vasc Surg 2013;57:464-474.e5.
- Tsai TT, Schlicht MS, Khanafer K, et al. Tear size and location impacts false lumen pressure in an ex vivo model of chronic type B aortic dissection. J Vasc Surg 2008;47:844-51. [PubMed]
- Loewe C, Czerny M, Sodeck GH, et al. A new mechanism by which an acute type B aortic dissection is primarily complicated, becomes complicated, or remains uncomplicated. Ann Thorac Surg 2012;93:1215-22. [PubMed]





