Best surgical option for arch extension of type B dissection: the endovascular approach
Introduction
Given the various clinical factors that must be considered, such as the period from onset and the presence of symptoms, the treatment of type B aortic dissections is extremely complicated. In general, aortic dissections are categorized as acute (the period within 14 days of onset) and chronic (the period more than 14 days after onset). Symptomatic cases (e.g., rupture, malperfusion, continuous pain, refractory hypertension) are classified as complicated, while those patients without such conditions are defined as uncomplicated. Complicated cases are currently treated with invasive techniques, while anti-hypertensive resting therapy is conventionally chosen for uncomplicated cases.
Despite advances in surgical techniques and postoperative management, the in-hospital mortality rate of patients undergoing conventional open surgical repair for acute complicated type B dissection ranges from 15% to 30%, with cerebrovascular complications and renal failure occurring in nearly 10% and 20%, respectively (1,2). In-hospital mortality increases to 63% for patients presenting with malperfusion and rupture (2). This has led to the development of a less invasive surgical procedure for acute type B aortic dissections called thoracic endovascular aortic repair (TEVAR) (2-6). Even in cases where TEVAR is indicated, the best treatment strategy and extent of repair required have not been well defined.
The endovascular approach for type B aortic dissections is to cover the entry tear and provide favorable aortic remodeling, thereby preventing early and late complications (2-5). The proximal entry tear of type B aortic dissection is usually located in the immediate vicinity of the orifice of the left subclavian artery (LSA). Given that the aim of TEVAR for type B dissections is primary entry closure, the proximal landing zone in the aortic arch must be secured. This requires hybrid surgery that involves the joint use of a surgical procedure; in particular, it requires debranching TEVAR that does not use extracorporeal circulation and involves reconstruction of cervical branches.
In this article, we describe the current status of this procedure for type B aortic dissections—in particular, debranching TEVAR for cases that need to extend to the aortic arch. We also discuss the prospects for this field and devices that will likely be approved in the future.
Device selection for debranching TEVAR of type B aortic dissections
We have been treating type B dissections since 1993 using self-made devices. Although early results were unsatisfactory due to device-related issues, technological innovations have facilitated a number of improvements and allowed for satisfactory outcomes (6). Currently, many ready-made devices exist (e.g., Gore TAG, Conformable TAG, Medtronic Valiant, Cook pro-form and Bolton Relay plus), however none are suitable for aortic dissections since they are limited by size variations, flexibility, and sufficient radial force.
In stent-graft procedures, particularly during the acute phase of disease, the expectation of restoring the thoracic area to its pre-dissection status (i.e., elimination of the false lumen), through occlusion of the entry tear, is high. In this setting, satisfactory acute and long-term outcomes have been also reported by the Stanford group (7).
What precautions should be taken in performing TEVAR for type B dissections? Two points are likely to be very important. One is the choice of the device size, and the other is where to place the proximal side of the device. In selecting the device, rigorous measurements are required. For dissecting aortic aneurysms, we select devices so that the proximal diameter of the stent-grafts is oversized by 10-20% of the native aorta and the distal diameter by 5-10%, in order to prevent a new intimal tear, both proximally and distally. For the same reason, bare stents are never used (6,8). Hence, we have to use either a tapered device or two devices with different diameters. Some reports have suggested that a straight device is sufficient for TEVAR for acute type B dissections. However, evidence regarding the durability of these devices is lacking. This underscores the need for durability data to consider device selection and treatment strategies.
With respect to the proximal landing zone, it is mandatory to deploy the stent-graft proximally in the non-dissected area. Presence of dissected aorta in the proximal side can lead to potentially fatal retrograde dissection and thereby necessitate an open procedure of ascending and arch repair. Given that the main entry tear in acute B dissections is often just below the LSA, safe treatment requires TEVAR to be deployed before the origin of the LSA, where the native aorta is assumed to be normal. Such a condition implies the covering of the LSA, and as consequence, debranching TEVAR becomes necessary (8-10).
Debranching TEVAR for type B aortic dissections
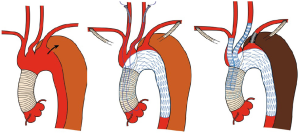
In TEVAR that requires aortic arch landing, the most important factor when choosing the technique is where the proximal landing zone can be fully secured. The techniques can be classified according to the proximal landing zone into cases in which: landing is possible in the distal arch (excluding the cervical branches; zone 3); only the LSA among the cervical branches is occluded (zone 2; Figure 1); cervical branches down to the left common carotid artery are occluded (zone 1; Figure 2); and cervical branches down to the brachiocephalic artery are occluded (zone 0; Figure 3). As previously discussed, using the normal native aorta as the proximal landing zone is crucial. The false lumen generally exists from just below the orifice of the LSA. Thus, zone 2 as the proximal landing zone is needed at the very least (9-11).
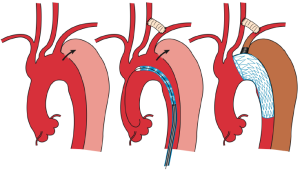
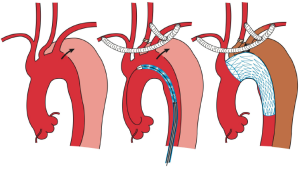
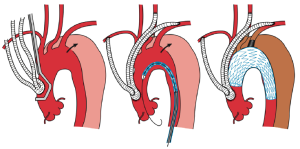
Usually, bypasses (debranching) are performed from either an open cervical branch or the ascending aorta to each of the occluded cervical arteries. As for debranching techniques (12-14), cervical artery transposition (12,13), and other procedures, none are as yet significantly satisfactory. Although several approaches have been described, each center should select the technique with which they have the most experience in order to secure high patency rates. For example, we usually perform the bypass from the right axillary artery to the left axially artery for zone 2 landing cases. While it can be argued that this bypass is long and bypass from left common carotid artery to the left axillary artery is more common, we have been using this approach to avoid left common carotid artery clamping and prevent stroke. Since 1997, optimal results of graft patency have been observed in over 1,000 cases.
For patients who require cervical debranching, the decision between staged or simultaneous procedures is considered on an individual basis, depending on the surgeon’s preference and surgical invasiveness. For those who require debranching TEVAR for zone 1 and 2 landing cases, we prefer to complete debranching and endovascular repair in the same setting. For zone 0 landing cases, the chest is still open and the total debranching procedure itself may be more invasive compared to zone 1 and 2 procedures. In such cases, TEVAR is typically performed on the following day. In our view, the rate of paraplegia after TEVAR depends on unstable blood pressure status. Thus, if the surgery is invasive, a staged procedure should be chosen.
With respect to LSA, the caliber of the vertebral arteries plays a central role. If the left vertebral artery is smaller to the right vertebral artery, according to magnetic resonance angiography (MRA), LSA occlusion may be sparingly performed. Nevertheless, several recent studies have shown that collateral circulation from subclavian arteries to the spine are crucial for preventing spinal cord ischemia (15). Thus, when possible, LSA bypass should be performed.
At present, little is known regarding the clinical outcomes of hybrid arch repair in patients with aortic dissection. A recent review of hybrid procedures for aortic arch dissections and other arch diseases found 27 studies in which 629 of 826 patients (76%) were treated by hybrid arch repair. The proportion of hybrid arch repairs performed in each study ranged from 23-100% (9). A review of literature describing hybrid arch repair in the context of aortic dissection identified only 12 studies in which 92 patients were treated for aortic dissection. Owing to the large heterogeneity of patients treated, it is difficult to compare or pool published results across studies (9). Kotelis et al. systematically reviewed a published series of hybrid arch repairs for various aortic diseases with stent grafting in zone 0 or 1, including 261 patients from 14 different studies (16). The in-hospital mortality rate was 6.5% (range, 0-15%), and stroke and paraplegia rates were 3.7% and 1.5% vs. 12% and 0% respectively. Another recent systematic review reported similar results (10).
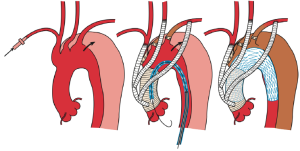
In terms of TEVAR for zone 0 landing cases, a recent review showed that hybrid aortic repair in zone 0 was associated with a mortality rate three-fold higher than repair involving zone 1 (9). Other studies have reported that debranching procedures with placement of a stent-graft along the curvature of the arch greatly increase the risk of severe adverse events when dealing with aortic dissections, particularly in acute cases where the aortic wall is very fragile and prone to damage by endovascular devices (14). Hopefully, this issue will likely resolve with the improvement of devices for debranching TEVAR. At present, devices lack the flexibility to adjust to the curvature of the arch and some devices have bare stents at the proximal side of the stent-grafts. Special devices for aortic dissections are clearly needed in the future, although at present, conformable TAG (cTAG) (W. L. Gore & Associates, Flagstaff, AZ, USA) (17) and Relay NBS plus (Bolton Medical, Inc., Sunrise, FL, USA) (18,19) may be suitable. Shirakawa et al. (11), reported achieving satisfactory early and mid-term results with TEVAR for zone 0 landing cases, which included aortic dissections. In order to avoid intraoperative complications, in particular retrograde aortic dissection, the authors used graft replacement of ascending aorta and total debranching TEVAR for zone 0 procedure (Figure 4). Given this strategy, no cases of retrograde aortic dissection were reported in the series. Moreover, the authors reported that side clamping of the ascending aorta for total debranching TEVAR for type B aortic dissections should be avoided, even when the ascending aorta is intact (11).
Retrograde dissection and stroke represent the Achilles’ heel of hybrid arch repairs. Retrograde aortic dissection following TEVAR occurs in 1-2% of cases (20-26). Owing to the lack of conformability of stent grafts in the aortic arch, the excessive radial forces applied at the convexity of the arch, and the need for aortic cross-clamping during total arch debranching and endovascular maneuvers within the arch, it is likely that retrograde aortic dissections are more frequent after hybrid arch procedures (20) than after single TEVAR. Another controversial issue related to this devastating complication is the use of proximal bare-spring stent-grafts as risk factor for retrograde aortic dissection (25). Cochennec et al. (14) reported that in four patients who presented with retrograde aortic dissections, a stent-graft with a proximal bare stent (Valiant device) or proximal hooks (Cook device) was deployed in the aortic arch. Retrograde aortic dissection usually requires emergency repair of the ascending aorta and aortic arch given its association with high mortality rates. This underscores the need to avoid retrograde dissection at all costs.
Fenestrated devices (27) and the chimney graft techniques (28) are frequently used alternatives for the debranching TEVAR procedure. Fenestrated devices (26) have holes in the cervical branch areas of the stent-graft, which sustain the blood flow to the cervical branches. These stent-grafts can also be used in combination with bypass (debranching), further reducing the invasiveness of the procedure. In particular, patients presenting with zone 0 landing cases may be managed in some cases without the need for thoracotomy. However, the possibility of endoleaks from fenestrations and the lack of long-term outcomes of these devices need to be considered.
The Chimney graft technique has traditionally been documented for treating chronic degenerative aneurysms and not for acute B dissections. By performing the chimney graft technique for both brachiocephalic and left common carotid arteries, surgery is minimally invasive and thoracotomy is not required, even when leaving the main device in place from zone 0. However, the overlap of the three devices in the proximal landing zone gives a gutter between the stent-grafts, leading in some cases to a type Ia endoleak, which prevents completion of aortic treatment. Furthermore, the occurrence of serious intraoperative and postoperative complications, especially stroke, has been reported (29,30). Given the fragility of the native aorta in type B aortic dissections, execution of such a risky procedure in an acute aortic scenario can be reckless. Because this approach represents a highly off-label use of stent-grafts, in our view it might be potentially indicated only for serious cases in which thoracotomy is impossible or during intra-operative bailout.
Prospects for hybrid procedures and devices
As discussed above, suitable TEVAR devices do not exist for type B aortic dissections. This has spurred the development of branched devices, that is, stent-grafts with branches, and has stimulated the point of clinical applicability as next-generation devices (28,31,32). Operations based on branched devices need small-diameter stent-grafts for cervical branches, which are inserted into the main stent-graft through tunnels for the supra-aortic trunks (Figures 5,6). Moreover, residual aortic dissection after graft replacement of ascending aorta for type A aortic dissections presents an ideal situation for the use of branch devices, particularly due to the difficulty of redo-surgery (32,33) (Figure 7). If such devices become commercially available in the future as ready-made devices, the chimney graft technique, which has been performed off-label, would become obsolete. Furthermore, given its invasiveness, debranching TEVAR would likely be shifted to TEVAR based on these branched devices.
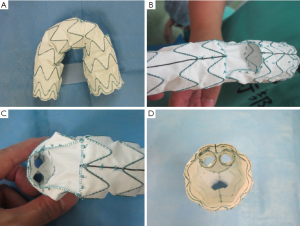
While current devices are fixed at the proximal and distal sides by the radial force of the stents, new experimental devices under evaluation enable fluid pressure bonding by a gel (e.g., injecting a polymer) (34) rather than a stent. Such a graft represents highly promising next-generation technology, and will potentially emerge in the future for thoracic aortic management, similar to device types that use a gel for abdominal aortic endografts. In patients with type B dissections who possess intrinsically weak vascular walls, the development of devices that do not rely solely on radial force is desirable. Although this may seem far-fetched, 20 years ago, stent-grafts did not exist, and 15 years ago, manufactured stent-grafts were entirely non-existent; as such, we have high hopes for the future.
In the coming years, there will be intense competition to develop new devices among types with similar characteristics (e.g., branched devices), improve delivery systems, and supplement devices with auxiliary functions (e.g., gels for fixation). We have high expectations for the next generations and how they will improve and advance treatment methods. We look forward to the future of hybrid surgery five years from now.
Acknowledgements
Disclosure: The author declares no conflict of interest.
References
- Bozinovski J, Coselli JS. Outcomes and survival in surgical treatment of descending thoracic aorta with acute dissection. Ann Thorac Surg 2008;85:965-70; discussion 970-1. [PubMed]
- Trimarchi S, Nienaber CA, Rampoldi V, et al. Role and results of surgery in acute type B aortic dissection: insights from the International Registry of Acute Aortic Dissection (IRAD). Circulation 2006;114:I357-64. [PubMed]
- Nienaber CA, Rousseau H, Eggebrecht H, et al. Randomized comparison of strategies for type B aortic dissection: the INvestigation of STEnt Grafts in Aortic Dissection (INSTEAD) trial. Circulation 2009;120:2519-28. [PubMed]
- Ehrlich MP, Dumfarth J, Schoder M, et al. Midterm results after endovascular treatment of acute, complicated type B aortic dissection. Ann Thorac Surg 2010;90:1444-8. [PubMed]
- Steuer J, Eriksson MO, Nyman R, et al. Early and long-term outcome after thoracic endovascular aortic repair (TEVAR) for acute complicated type B aortic dissection. Eur J Vasc Endovasc Surg 2011;41:318-23. [PubMed]
- Kato M, Matsuda T, Kaneko M, et al. Outcomes of stent-graft treatment of false lumen in aortic dissection. Circulation 1998;98:II305-11; discussion II311-2.
- Dake MD, Kato N, Mitchell RS, et al. Endovascular stent-graft placement for the treatment of acute aortic dissection. N Engl J Med 1999;340:1546-52. [PubMed]
- Shigemura N, Kato M, Kuratani T, et al. New operative method for acute type B dissection: left carotid artery-left subclavian artery bypass combined with endovascular stent-graft implantation. J Thorac Cardiovasc Surg 2000;120:406-8. [PubMed]
- Cao P, De Rango P, Czerny M, et al. Systematic review of clinical outcomes in hybrid procedures for aortic arch dissections and other arch diseases. J Thorac Cardiovasc Surg 2012;144:1286-300, 1300.e1-2.
- Antoniou GA, El Sakka K, Hamady M, et al. Hybrid treatment of complex aortic arch disease with supra-aortic debranching and endovascular stent graft repair. Eur J Vasc Endovasc Surg 2010;39:683-90. [PubMed]
- Shirakawa Y, Kuratani T, Shimamura K, et al. The efficacy and short-term results of hybrid thoracic endovascular repair into the ascending aorta for aortic arch pathologies. Eur J Cardiothorac Surg 2014;45:298-304; discussion 304. [PubMed]
- Cinà CS, Safar HA, Laganà A, et al. Subclavian carotid transposition and bypass grafting: consecutive cohort study and systematic review. J Vasc Surg 2002;35:422-9. [PubMed]
- Czerny M, Pfannmüller B, Borger MA, et al. Hybrid debranching technique for aortic arch replacement. Multimed Man Cardiothorac Surg 2011;2011:mmcts.2011.005108.
- Cochennec F, Tresson P, Cross J, et al. Hybrid repair of aortic arch dissections. J Vasc Surg 2013;57:1560-7. [PubMed]
- Eagleton MJ, Shah S, Petkosevek D, et al. Hypegastric and subclavian artery patency affects onset and recovery of spinal cord ishemia associated with aortic endografting. J Vasc Surg 2014;59:89-94. [PubMed]
- Kotelis D, Geisbüsch P, Attigah N, et al. Total vs hemi-aortic arch transposition for hybrid aortic arch repair. J Vasc Surg 2011;54:1182-1186.e2.
- Georg Y, Schwein A, Lejay A, et al. Practical experience with the TAG and conformable TAG devices: lessons learned in about 100 cases. J Cardiovasc Surg (Torino) 2013;54:605-15. [PubMed]
- Oberhuber A, Winkle P, Schelzig H, et al. Technical and clinical success after endovascular therapy for chronic type B aortic dissections. J Vasc Surg 2011;54:1303-9. [PubMed]
- Zipfel B, Czerny M, Funovics M, et al. Endovascular treatment of patients with types A and B thoracic aortic dissection using Relay thoracic stent-grafts: results from the RESTORE Patient Registry. J Endovasc Ther 2011;18:131-43. [PubMed]
- Geisbüsch P, Kotelis D, Müller-Eschner M, et al. Complications after aortic arch hybrid repair. J Vasc Surg 2011;53:935-41. [PubMed]
- Hughes GC, Daneshmand MA, Balsara KR, et al. “Hybrid” repair of aneurysms of the transverse aortic arch: midterm results. Ann Thorac Surg 2009;88:1882-7; discussion 1887-8.
- Fattori R, Tsai TT, Myrmel T, et al. Complicated acute type B dissection: is surgery still the best option?: a report from the International Registry of Acute Aortic Dissection. JACC Cardiovasc Interv 2008;1:395-402. [PubMed]
- White RA, Miller DC, Criado FJ, et al. Report on the results of thoracic endovascular aortic repair for acute, complicated, type B aortic dissection at 30 days and 1 year from a multidisciplinary subcommittee of the Society for Vascular Surgery Outcomes Committee. J Vasc Surg 2011;53:1082-90. [PubMed]
- Thrumurthy SG, Karthikesalingam A, Patterson BO, et al. A systematic review of mid-term outcomes of thoracic endovascular repair (TEVAR) of chronic type B aortic dissection. Eur J Vasc Endovasc Surg 2011;42:632-47. [PubMed]
- Eggebrecht H, Thompson M, Rousseau H, et al. Retrograde ascending aortic dissection during or after thoracic aortic stent graft placement: insight from the European registry on endovascular aortic repair complications. Circulation 2009;120:S276-81. [PubMed]
- Fattori R, Lovato L, Buttazzi K, et al. Extension of dissection in stent-graft treatment of type B aortic dissection: lessons learned from endovascular experience. J Endovasc Ther 2005;12:306-11. [PubMed]
- Kitagawa A, Greenberg RK, Eagleton MJ, et al. Fenestrated and branched endovascular aortic repair for chronic type B aortic dissection with thoracoabdominal aneurysms. J Vasc Surg 2013;58:625-34. [PubMed]
- Moulakakis KG, Mylonas SN, Dalainas I, et al. The chimney-graft technique for preserving supra-aortic branches: a review. Ann Cardiothorac Surg 2013;2:339-46. [PubMed]
- Hogendoorn W, Schlösser FJ, Moll FL, et al. Thoracic endovascular aortic repair with the chimney graft technique. J Vasc Surg 2013;58:502-11. [PubMed]
- Moulakakis KG, Mylonas SN, Dalainas I, et al. The chimney-graft technique for preserving supra-aortic branches: a review. Ann Cardiothorac Surg 2013;2:339-46. [PubMed]
- Chuter TA, Schneider DB, Reilly LM, et al. Modular branched stent graft for endovascular repair of aortic arch aneurysm and dissection. J Vasc Surg 2003;38:859-63. [PubMed]
- Botta L, Fratto P, Cannata A, et al. Aortic-arch Reconstruction with Bolton Medical Branched Thoracic Stent Graft. EJVES Extra 2013;25:e38-e41.
- Kuratani T, Shirakawa Y, Shimamura K, et al. Total Endovascular Repair of an Enlarged Residual Aortic Dissection With Bolton Medicalra 2013;Vasc Surg 2003;38:85ascular Today 2014. [Epub ahead of print].
- Mehta M, Valdés FE, Nolte T, et al. One-year outcomes from an international study of the Ovation Abdominal Stent Graft System for endovascular aneurysm repair. J Vasc Surg 2014;59:65-73.e1-3.






