Sutureless aortic valve replacement: a systematic review and meta-analysis
Introduction
Aortic valve stenosis is the most common valve disease, resulting in a prognosis of 30-50% mortality at one-year follow-up without intervention for severe and symptomatic cases (1,2). Currently, the conventional treatment of severe aortic valve disease is surgical aortic valve replacement (AVR) through a median sternotomy, with complications and mortality decreasing in recent years (3). However, in an era transformed by an aging population, the presenting patient is increasingly older and sicker with heavily calcified valves, root calcification and with diffuse atherosclerosis and diabetes (4). This modern surgical challenge has triggered the development of less invasive procedures, assumed to diminish the operative risk. Thus, recent advances in technologies have led to the introduction of alternative treatment modalities including sutureless AVR (SU-AVR).
As a cardiac valve substitute, sutureless prostheses reduce the need for sutures after annular decalcification, thereby reducing aortic cross-clamp and cardiopulmonary bypass (CPB) duration and facilitating a minimally invasive approach. While there is current data supporting reduced surgical operative times with SU-AVR (5,6), whether the use of this technology results in improved clinical outcomes remains uncertain. The present systematic review and meta-analysis aims to identify and analyze the available evidence on the safety, clinical efficacy and complications of sutureless valves for AVR.
Methods
Literature search strategy
Electronic searches were performed using Ovid Medline, PubMed, Cochrane Central Register of Controlled Trials (CCTR), Cochrane Database of Systematic Reviews (CDSR), ACP Journal Club, and Database of Abstracts of Review of Effectiveness (DARE) from their dates of inception to January 2014. To achieve the maximum sensitivity of the search strategy, we combined the terms: “sutureless” AND “aortic valve” AND “surgery OR operation OR replacement” as either key words or MeSH terms. The reference lists of all retrieved articles were reviewed for further identification of potentially relevant studies, assessed using the inclusion and exclusion criteria. Expert academic cardiothoracic surgeons (Marco Di Eusanio, Tristan D. Yan) were consulted as to whether they knew of any unpublished data.
Selection criteria
Eligible studies for the present systematic review and meta-analysis included those in which patient cohorts underwent AVR using a sutureless valve such as Perceval S (Sorin Group, Saluggia), 3F Enable (ATS Medical, Minneapolis), Trilogy (Arbor Surgical Technologies, California) or Edwards Intuity (Edwards Lifesciences, California). Studies that did not include mortality or complications as endpoints were excluded. When institutions published duplicate studies with accumulating numbers of patients or increased lengths of follow-up, only the most complete reports were included for quantitative assessment at each time interval. All publications were limited to those involving human subjects and in the English language. Abstracts, case reports, conference presentations, editorials, reviews and expert opinions were excluded.
Data extraction and critical appraisal
All data were extracted from article texts, tables and figures. Two investigators independently reviewed each retrieved article (K.P., Y.C.T.). Discrepancies between the two reviewers were resolved by discussion and consensus. If the study provided medians and interquartile ranges instead of means and SDs, we imputed the means and SDs as described by Hozo et al. (7). Because quality scoring is controversial in meta-analyses of observational studies, two reviewers (K.P., Y.C.T.) independently appraised each article included in our analysis according to a critical review checklist of the Dutch Cochrane Centre proposed by MOOSE (8). The key points of this checklist include: (I) clear definition of study population; (II) clear definition of outcomes and outcome assessment; (III) independent assessment of outcome parameters; (IV) sufficient duration of follow-up; (V) no selective loss during follow-up; and (VI) important confounders and prognostic factors identified. The final results were reviewed by senior investigators (M.D.E., T.D.Y.).
Statistical analysis
A meta-analysis of proportions was conducted for the available main perioperative and postoperative variables. Firstly, to establish variance of raw proportions, a Freeman-Tukey transformation was applied (9). To incorporate heterogeneity (anticipated among the included studies), transformed proportions were combined using DerSimonian-Laird random effects models (10). Finally the pooled estimates were back-transformed. Heterogeneity was evaluated using Cochran Q and I2 test. Weighted means were calculated by determining the total number of events divided by total sample size. Weighted Pearson’s coefficient (rs) was used to calculate correlation coefficients for meta-regression analysis of outcomes based on midpoint of study periods. All analyses were performed using the metafor package for R version 3.01. P values <0.05 were considered statistically significant.
Evidence of publication bias was sought using Begg methods. Contour-enhanced funnel plot was performed to aid in interpretation of the funnel plot. Possible asymmetry was investigated using trim-and-fill analysis.
Results
Quality of studies
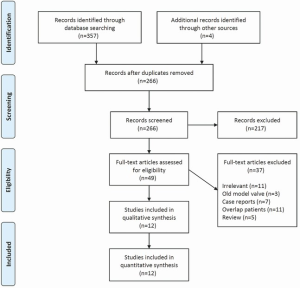
A total of 361 studies were identified through six electronic database searches and from other sources such as reference lists (Figure 1). After exclusion of duplicate or irrelevant references, 46 potentially relevant articles were retrieved. After detailed evaluation of these articles, 12 studies remained for assessment, including a total of 1,037 patients undergoing SU-AVR.
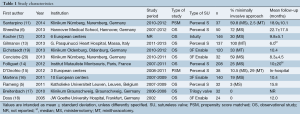
All of the included 12 studies were observational studies, with 10 prospective (5,6,11-18), 2 retrospective (19,20) and 2 propensity-matched studies (11,15) (Table 1). There were 7 studies (6,11-14,16,19) which consisted of 50 or more patients undergoing AVR with a sutureless valve, while the remaining 5 studies had fewer than 50 patients (5,15,17,18,20). The Perceval S valve (n=502) was used in 6 studies (5,6,11,13,15,21), the 3F Enable valve (n=316) used in 4 studies (16,18-20), Trilogy valve (n=32) (17) and Edwards Intuity valve (n=146) used in one study (12) each.
Full table
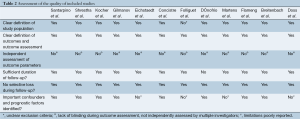
Only 5 studies reported mean follow-up equal or greater than 12 months (5,6,11,18,21). One study (14) reported follow-up up to 4 years. Another study confined analysis only to hospital outcomes (15). 30-day mortality was reported in all studies except Doss et al. (18), while postoperative mortality at follow-up was reported in all studies except D’Onofrio et al. (15). The quality assessment of each included study is presented in Table 2.
Full table
Patients’ characteristics
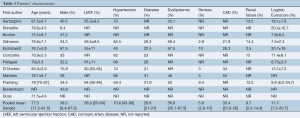
Overall, 39% of patients were male, with a weighted mean age of 77.3 (range, 71.5-81.5) years. The mean LVEF for included patients was 58.9% (range, 55-64%) with weighted pooled logistic Euroscore of 11.7 (range, 7.5-20.7). The majority of patients had hypertension (70.6%; range, 45-86%) while 26.6%, 35.4% and 56.9% of included patients had diabetes, coronary artery disease and dyslipidemia, respectively. A smaller fraction of patients had chronic lung disease (14.3%; range, 12.5-18.9%), prior strokes (5.8%; range, 2.9-10%) and renal failure (9.7%; range, 2.5-14.4%). Other comorbidities such as atrial fibrillation, mitral and tricuspid insufficiency, and peripheral vascular disease were poorly reported in three or fewer studies. Baseline characteristics are summarized in Table 3.
Full table
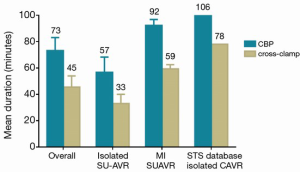
Weighted pooled estimates of CPB and cross-clamp time were 73.1 minutes [95% confidence interval (CI), 63.2-83.1 minutes; I2 =97%; P<0.001] and 46.5 minutes (95% CI, 38.9-54.0 minutes; I2 =98%; P<0.001), respectively. For isolated AVR, CPB and cross-clamp were 56.7 minutes (95% CI, 45.2-68.2 minutes; I2 =98%; P<0.001) and 33.1 minutes (95% CI, 25.5-40.8 minutes; I2 =99%; P<0.001), respectively, with significant heterogeneity detected.
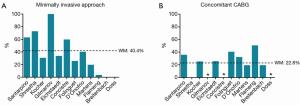
A subgroup analysis suggested that cross-clamp duration was comparable for full sternotomy (WM, 53.6; 95% CI, 45.6-91.6; n=3) versus minimally invasive SU-AVR (WM, 59.3; 95% CI, 56.1-62.4; n=1). CBP had a trend towards being lower with full sternotomy (WM, 78.2; 95% CI, 14.5-141.9; n=2) versus minimally invasive approach (WM, 92.3; 95% CI, 87.7-96.8; n=1). Operative characteristics are summarized in Table 4 and Figures 2 and 3.
Full table
Assessment of safety
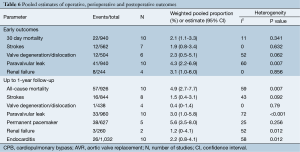
From ten studies, mortality incidence was 2.1% (95% CI, 1.1-3.3%; I2 =11%; P=0.341) at 30 days, and 4.9% at 1 year (95% CI, 2.7-7.7%; I2 =59%; P=0.007; Figure 4A). There was similar incidence of neurological events at early follow-up (1.9%; 95% CI, 0.8-3.4%; I2 =0%; P=0.632; Figure 4B) and later follow-up (1.5%; 95% CI, 0.4-3.1%; I2 =43%; P=0.092). Weighted pooled estimates of renal failure, endocarditis and reoperation for bleeding were 1.2% (95% CI, 0-4.1%; I2 =52%; P=0.012), 2.2% (95% CI, 0.8-4.1%; I2 =58%; P=0.012; Figure 4C) and 1.4% (95% CI, 0.1-3.6%; I2 =52%; P=0.103), respectively.
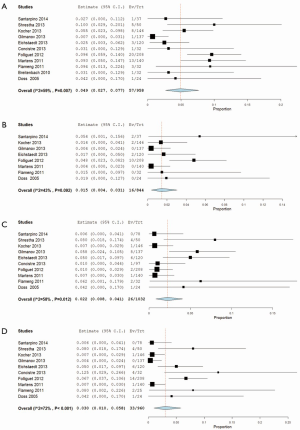
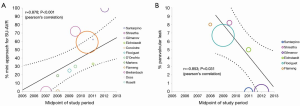
Post-operative paravalvular leakage was reported by ten studies to be 3.0% (95% CI, 1.0-5.8%; I2 =72%; P<0.001; Figure 4D). Weighted pooled estimates of structural valve deterioration and permanent pacemaker implantation were 0.4% (95% CI; 0-1.4%; I2 =0%; P=0.79) and 5.6% (95% CI, 3.5-8.0%; I2 =25%; P=0.252), respectively. The midpoint of study periods for Perceval S valve studies negatively correlated with incidence of paravalvular leakage (r=–0.853; P=0.031, Pearson’s correlation) (Figure 5).
Assessment of hemodynamic outcomes
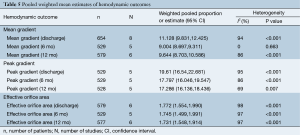
Mean gradient at discharge and 12 month follow-up were reported in 8 and 6 studies, respectively. Pooled weighted estimate of mean gradient was 11.13 mmHg (95% CI, 9.8-12.4 mmHg, I2 =94%; P<0.001) at discharge, 9.0 mmHg (95% CI, 8.7-9.3 mmHg; I2 =0%; P=0.663) at 6 months and 9.6 mmHg (95% CI, 8.7-10.6 mmHg; I2 =86%; P<0.001) at 12 month follow-up (Table 5).
Full table
Peak gradient was reported in five studies at discharge, 6 and 12 month follow-up. Pooled weighted estimate of peak gradient was 19.6 mmHg (95% CI, 16.5-22.7 mmHg, I2 =95%; P<0.001) at discharge, 17.8 mmHg (95% CI, 16.0-19.5 mmHg; I2 =86%; P<0.001) at 6 months and 17.3 mmHg (95% CI, 16.1-18.4 mmHg; I2 =69%; P=0.007) at 12 month follow-up.
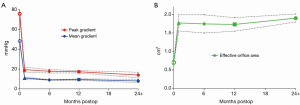
The effective orifice area was similar at discharge (1.77 cm2, 95% CI, 1.6-2.0 cm2; I2 =98%; P<0.001), 6 month (1.75 cm2, 95% CI, 1.5-2.0 cm2; I2 =97%; P<0.001) and 12 month (1.73 cm2, 95% CI, 1.5-1.9 cm2; I2 =97%; P<0.001) follow-up. Significant heterogeneity was detected in all hemodynamic outcomes at discharge and 12-month follow-up. Hemodynamic outcomes are summarized in Table 6 and Figure 6.
Full table
Publication bias
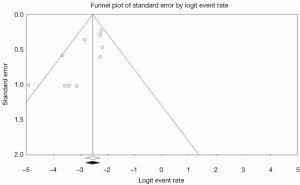
Inspection of the funnel plot (Figure S1) did not show significant asymmetry for all-cause. Trim-and-fill analysis indicated that no studies were missing. Publication bias was not significant, with Begg’s test score of P=0.2429 (tau =–0.2778, z =1.1676). These results suggest that publication bias was not a significant influencing factor.
Discussion
Aortic valve stenosis is emerging as the most common heart disease in Western countries due to a rapidly aging population, yet adequate treatment remains a crucial clinical challenge, especially in mid-high risk patients (1). In order to minimize mortality and to expand the indication of surgical treatment for high-risk patients who are otherwise inoperable, less invasive alternative approaches using innovative technologies have been developed and are increasingly used (15,22). Transcatheter aortic valve implantation (TAVI) and SU-AVR represent two important advances in the treatment of aortic valve disease, and are likely to revolutionize valve therapy in the near future.
Similar to conventional AVR, SU-AVR requires valve excision and annular decalcification, but avoids the use of permanent sutures at the decalcified annulus. Thus, the rationale for its use lies in its potential to reduce operative trauma by decreasing operative times and facilitating minimally invasive approaches (13).
In cardiac surgery, prolonged CPB and cross-clamp durations are strong independent risk factors for post-operative mortality and morbidity (23,24). Their detrimental effect becomes further amplified when operations are performed in patients burdened by advanced age and other serious comorbidities. By avoiding the placement and tying of sutures, SU-AVR has resulted in shortened CPB and cross-clamp times in multiple studies, with Flameng et al. reporting CPB and cross-clamp durations of 46 and 22 minutes, respectively (5). In the current meta-analysis of 12 observational studies, CPB and cross-clamp durations were 73 and 45 minutes, respectively, and were further shortened for stand-alone AVR procedures being 57 and 33 minutes, respectively. This data favorably compares with most recent data for isolated AVR with full sternotomy from the STS database (25) showing CPB and cross clamp times of 106 and 78 minutes, respectively.
Reduced duration of cross-clamp and CPB during AVR with sutureless valves may further promote AVR with or without concomitant cardiac surgery, which otherwise would not be suitable for high risk patients undergoing long cardiac procedures. Moreover, with sutureless valves, the CPB and cross-clamp duration can be further reduced in minimally invasive AVR (11,16,20,26,27). Indeed, a subgroup analysis suggested similar cross-clamp durations for both full sternotomy (WM, 53.6 minutes) and minimally invasive (WM, 59.3 minutes) approaches. This observation can be explained by the fact that sutureless valve technology is likely to be embraced by surgeons with more extensive experience on minimally invasive cardiac surgery (MICS), but also indicates that sutureless valves facilitate MICS remarkably. The significant correlation between the increased use of minimally invasive incisions in SU-AVR and midpoint of study periods strongly support this notion (Figure 5A). It is in complex operations and high-risk patients that sutureless valves are maximally appreciated.
The hemodynamic performance of sutureless valves is another important determinant of their efficacy in patients with aortic valve stenosis. Reduced mean and peak gradients and enhanced transvalvular flow and effective orifice area are indicative of efficacious intervention via a sutureless approach. Reports of mid-term and long-term hemodynamic performance beyond 4 years have been scarce, and therefore the current meta-analysis focused on short-term performance. Sadowski et al. (28) reported maximal and mean gradients of 11.6 and 6.8 mmHg, respectively on discharge. These echocardiographic parameters progressively decreased to 10.1 and 5.2 mmHg at 4 years follow-up, supporting the efficacy of the 3F Enable sutureless valve at short- and mid-term follow-up. These results were similar to the pooled estimates of the current meta-analysis, which reported mean gradients to be decreased significantly from 48.5 mmHg preoperatively to 9.4 mmHg at 1-year follow-up and 8 mmHg at 2-year follow-up. Pooled effective orifice area also increased from 0.7 cm2 preoperatively to 1.9 cm2 at 2-year follow-up, constituting over a 2-fold increase in area. While long-term durability and hemodynamic data is currently lacking, sutureless valves appear to have excellent hemodynamic parameters at perioperative and short-term follow-up.
While SU-AVR appears to facilitate minimally invasive surgery, shorten cross-clamp and CPB duration, and provide excellent valve hemodynamics, whether this translates into improved clinical outcomes is still not well established (29). In the largest prospective, multicenter series including 208 high-risk patients implanted with the Perceval S sutureless valve and followed up to 4 years (14), the reported in-hospital and 1-year mortality rates were 2.4% and 12.9%, respectively. Similar mortality rates were described by Kocher et al., who presented results from 146 patients implanted with the Edwards Intuity sutureless valve (12). The mortality rates at 30-day and 1-year were 2.1% and 7.5% respectively, with 30.8% of patients undertaking a minimally invasive approach for ministernotomy or minithoracotomy. These low mortality rates are supported by the current meta-analysis, with pooled estimates of 30-day and 1-year mortality rates being 2.1% and 5.1% respectively, equivalent to the mortality rates reported recently for surgical AVR. While the above findings are limited by the lack of long-term evidence and randomized comparisons of SU-AVR versus surgical AVR, the evidence to date indicates low and acceptable mortality rates for SU-AVR in the short-term.
In the current study, pooled stroke incidences (1.4%; range, 0-4.8%) appeared to be comparable to available evidence in the literature for conventional AVR (3). As such, the current evidence demonstrates acceptable rates of neurological events for sutureless valves. However, future randomized studies of longer follow-up and larger sample sizes are required to draw definitive conclusions.
Incidence of valve deterioration and dislocation was low, with 2.3% and 0.4% incidence at perioperative and postoperative follow-up, respectively. In contrast to previous studies (20) with reports of up to 12.5%, the pooled results from the current systematic review indicates lower paravalvular leaks (PVL) rates of 2-4% at follow-up. This complication may be a function of the learning curve involved in the introduction of this innovative surgical technique. It is possible that PVL adverse events may be reduced with experience (Figure 5B). Pooled estimates of permanent pacemaker implantations for sutureless valves were satisfactory (5.6%), comparable to pooled estimates of 3.0% for conventional AVR and lower than that for TAVI (13.2%) reported in a recent systematic review (30). Overall, data from the current meta-analysis suggests that sutureless valve implantation has comparable complication rates to surgical AVR. However, further studies are required to confirm whether this is the case at long-term follow-up.
The present findings are limited by several constrains. Multiple outcomes were not adequately reported, including resource-related outcomes such as intensive care unit stay, hospitalization duration, cost-effectiveness and quality of life outcomes. Such parameters are also of critical importance when considering SU-AVR as an alternative to conventional AVR and TAVI. The lack of randomization, blinding and comparators in the included studies indicates an inherent source of unaccounted bias, which may have skewed the presented results. Given the small sample sizes of each study with lack of statistical power and randomization, complication rates may have been underemphasized. Another major limitation of the current evidence base is the absence of long-term data beyond 4 years. The durability and long-term complications of sutureless valves could not be assessed, hence limiting the provision of evidence-based guidelines and recommendations. Long-term studies are also required to compare SU-AVR with conventional AVR and TAVI approaches, particularly in the setting of high-risk patients, to determine whether SU-AVR and TAVI are safe and efficacious, and which approach offers more clinical advantages for each individual patient. Finally, there was significant heterogeneity in outcomes such as PVL and valve degeneration, which may reflect the varying degrees of technical experience between individual institutions and the divergent efficacy and safety between different types of sutureless valve types.
Conclusions
In summary, sutureless valves provide the possibility of AVR with shortened CPB and cross-clamp times, thereby facilitating minimally invasive approaches as well as concomitant cardiac surgery for high-risk patients. Current short-term clinical evidence indicates similar mortality and complication rates compared to conventional AVR, with satisfactory hemodynamic performance. Long-term follow-up data, adequately powered sample sizes and future randomized studies and registry data are required to adequately assess the durability and long-term complications of SU-AVR.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Vahanian A, Otto CM. Risk stratification of patients with aortic stenosis. Eur Heart J 2010;31:416-23. [PubMed]
- Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597-607. [PubMed]
- Brown JM, O’Brien SM, Wu C, et al. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database. J Thorac Cardiovasc Surg 2009;137:82-90. [PubMed]
- Frilling B, von Renteln-Kruse W, Riess FC. Evaluation of operative risk in elderly patients undergoing aortic valve replacement: the predictive value of operative risk scores. Cardiology 2010;116:213-8. [PubMed]
- Flameng W, Herregods MC, Hermans H, et al. Effect of sutureless implantation of the Perceval S aortic valve bioprosthesis on intraoperative and early postoperative outcomes. J Thorac Cardiovasc Surg 2011;142:1453-7. [PubMed]
- Shrestha M, Maeding I, Höffler K, et al. Aortic valve replacement in geriatric patients with small aortic roots: are sutureless valves the future? Interact Cardiovasc Thorac Surg 2013;17:778-82; discussion 782. [PubMed]
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [PubMed]
- Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008-12. [PubMed]
- Freeman MF, Tukey JW. Transformations Related to the Angular and the Square Root. The Annals of Mathematical Statistics 1950;21:607-11.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. [PubMed]
- Santarpino G, Pfeiffer S, Jessl J, et al. Sutureless replacement versus transcatheter valve implantation in aortic valve stenosis: a propensity-matched analysis of 2 strategies in high-risk patients. J Thorac Cardiovasc Surg 2014;147:561-7. [PubMed]
- Kocher AA, Laufer G, Haverich A, et al. One-year outcomes of the Surgical Treatment of Aortic Stenosis With a Next Generation Surgical Aortic Valve (TRITON) trial: a prospective multicenter study of rapid-deployment aortic valve replacement with the EDWARDS INTUITY Valve System. J Thorac Cardiovasc Surg 2013;145:110-5; discussion 115-6. [PubMed]
- Gilmanov D, Miceli A, Bevilacqua S, et al. Sutureless implantation of the perceval s aortic valve prosthesis through right anterior minithoracotomy. Ann Thorac Surg 2013;96:2101-8. [PubMed]
- Folliguet TA, Laborde F, Zannis K, et al. Sutureless perceval aortic valve replacement: results of two European centers. Ann Thorac Surg 2012;93:1483-8. [PubMed]
- D’Onofrio A, Messina A, Lorusso R, et al. Sutureless aortic valve replacement as an alternative treatment for patients belonging to the “gray zone” between transcatheter aortic valve implantation and conventional surgery: a propensity-matched, multicenter analysis. J Thorac Cardiovasc Surg 2012;144:1010-6. [PubMed]
- Martens S, Sadowski J, Eckstein FS, et al. Clinical experience with the ATS 3f Enable® Sutureless Bioprosthesis. Eur J Cardiothorac Surg 2011;40:749-55. [PubMed]
- Breitenbach I, Wimmer-Greinecker G, Bockeria LA, et al. Sutureless aortic valve replacement with the Trilogy Aortic Valve System: multicenter experience. J Thorac Cardiovasc Surg 2010;140:878-84, 884.e1.
- Doss M, Martens S, Wood JP, et al. Aortic leaflet replacement with the new 3F stentless aortic bioprosthesis. Ann Thorac Surg 2005;79:682-5; discussion 685. [PubMed]
- Eichstaedt HC, Easo J, Härle T, et al. Early single-center experience in sutureless aortic valve implantation in 120 patients. J Thorac Cardiovasc Surg 2014;147:370-5. [PubMed]
- Concistrè G, Santarpino G, Pfeiffer S, et al. Two alternative sutureless strategies for aortic valve replacement: a two-center experience. Innovations (Phila) 2013;8:253-7. [PubMed]
- Folliguet TA, Laborde F, Zannis K, et al. Sutureless perceval aortic valve replacement: results of two European centers. Ann Thorac Surg 2012;93:1483-8. [PubMed]
- Lorusso R, Gelsomino S, Renzulli A. Sutureless aortic valve replacement: an alternative to transcatheter aortic valve implantation? Curr Opin Cardiol 2013;28:158-63. [PubMed]
- Salis S, Mazzanti VV, Merli G, et al. Cardiopulmonary bypass duration is an independent predictor of morbidity and mortality after cardiac surgery. J Cardiothorac Vasc Anesth 2008;22:814-22. [PubMed]
- Al-Sarraf N, Thalib L, Hughes A, et al. Cross-clamp time is an independent predictor of mortality and morbidity in low- and high-risk cardiac patients. Int J Surg 2011;9:104-9. [PubMed]
- Miceli A, Santarpino G, Pfeiffer S, et al. Minimally invasive aortic valve replacement with Perceval S sutureless valve: Early outcomes and one-year survival from two European centers. J Thorac Cardiovasc Surg 2014;148:2838-43. [PubMed]
- Santarpino G, Giardina S, Pollari F, et al. Cost Saving after Sutureless Replacement in Aortic Valve Stenosis: Results from a Propensity-Matched Score Analysis in Germany. Value in Health 2013;16:A520.
- Martens S, Zierer A, Ploss A, et al. Sutureless Aortic Valve Replacement via Partial Sternotomy. Innovations (Phila) 2010;5:12-5. [PubMed]
- Sadowski J, Kapelak B, Pfitzner R, et al. Sutureless aortic valve bioprothesis ‘3F/ATS Enable’--4.5 years of a single-centre experience. Kardiol Pol 2009;67:956-63. [PubMed]
- Santarpino G, Pfeiffer S, Concistré G, et al. The Perceval S aortic valve has the potential of shortening surgical time: does it also result in improved outcome? Ann Thorac Surg 2013;96:77-81; discussion 81-2. [PubMed]
- Cao C, Ang SC, Indraratna P, et al. Systematic review and meta-analysis of transcatheter aortic valve implantation versus surgical aortic valve replacement for severe aortic stenosis. Ann Cardiothorac Surg 2013;2:10-23. [PubMed]





