Pitfalls in the hybrid approach of type B aortic dissection with arch involvement
Introduction
Type B aortic dissection (TBAD) with arch involvement is a severe, life-threatening condition. By combining open surgical and endovascular techniques, the hybrid approach has emerged as the preferred treatment option for this challenging disease. The hybrid concept entails reimplantation or bypass of all epiaortic vessels to create an adequate proximal landing zone suitable for thoracic endovascular aortic repair (TEVAR). However, the outcome of patients with TBAD treated with complete surgical debranching in the native ascending aorta and subsequent TEVAR is unsatisfactory, resulting in a mortality rate of 27-70% (1,2).
Consequently, the therapeutic management of complicated TBAD by open arch replacement with frozen elephant trunk (FET) placement is becoming the first line treatment in many leading centers for aortic surgery.
Pitfalls in beating heart total arch rerouting
Complete or partial, beating-heart, supra-aortic debranching associated with TEVAR has been considered an alternative solution to open surgery in TBAD with arch involvement. However, recent reviews showed unsatisfactory results with high mortality, especially when the repair involved the Criado’s zone zero (3). There are multiple reasons for these disappointing results.
Retrograde dissection
Retrograde aortic dissection is an important complication after stenting of the distal dissection, with an incidence of 1-3% (3). This finding is not surprising, given the underlying aortic substrate. Causes of this adverse event include clamp damage at the ascending aorta, stiff guide-wire/nose cone manipulation in the aortic arch during TEVAR procedure, and spontaneous dissection progression in the ascending aorta. This complication necessitates emergent open surgical conversion with total arch replacement. In such cases, cross-clamping of ascending aortas with stent-grafts may be feasible with an extra-strong atraumatic cross-clamp. In some types of endografts (Cook or Medtronic), it is necessary to remove the bare metal stents with wire-cutters in order to perform a hemostatic suture between the vascular prosthesis and the stent graft.
Stroke
Perioperative stroke is related to embolism formation during the de-branching procedure or excessive manipulation in the dissected aortic arch during TEVAR. It is reported that stroke rates range from 0.8-18% (4). Care must be taken during the procedure to minimize these risks.
Bypass occlusion and stenosis
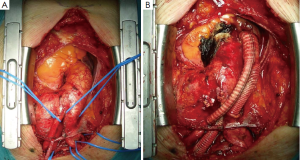
Bypass occlusion or stenosis is a rare complication usually related to the position of the Dacron branches arising from the ascending aorta. It is important to respect the appropriate distance between the proximal portion of the debranching graft and the posterior face of the sternum, as determined from the right lateral position of the ascending aortic anastomosis (Figure 1A,B).
Endoleak
Proximal type I endoleak is the most common indication for secondary surgical or endovascular intervention (5). These complications are often caused by a shorter-than-recommended proximal landing zone (<2 cm) or a difficult endograft conformability at the inner curve of the aortic arch (bird beak sign). Aortic rupture after perforation of the outer adventitial layer of the aortic wall has also been reported (2).
Pitfalls in hybrid arch repair with FET
It must be kept in mind that type A dissection could easily develop when treating a dissected arch. Based on our and other leading centers’ experiences, we believe that the best approach to manage a “complicated” type B dissection extending to the aortic arch may be an open arch rerouting with frozen elephant trunk (FET). The studies regarding such scenarios using FET show mortalities between 10-30%, which is significantly better than the poor results related to “beating heart” hybrid aortic arch repair (6).
The FET is a complex procedure that our center has used since 2005. With this single-stage approach, using the E-vita Open Plus (Jotec GmbH, Hechingen, Germany), it is possible to stabilize the first portion of dissected descending thoracic aorta and produce a complete correction or create a safe and straight landing zone for subsequent TEVAR in the residual dissected downstream aorta (7). Understandably, the FET procedure also demonstrates some adverse events related to difficult management of technical aspects.
Set-up
The classic median sternotomy is performed with extension of the incision superiorly along the median border of the sternocleidomastoid muscle in order to effectively expose all epiaortic vessels, especially the left subclavian artery (LSA), which may be more difficult to approach surgically (Figure 2).
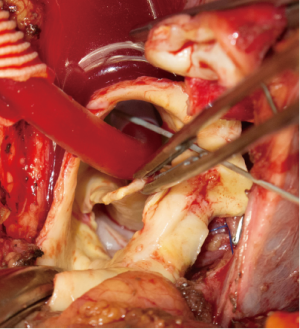
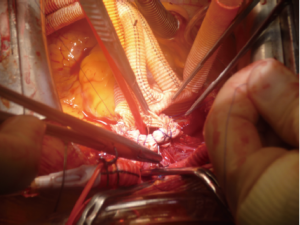
For arterial systemic perfusion, we believe that the most efficient sites of cannulation are the right axillary artery (exposed through a small subclavicular incision) or the innominate trunk (through the median sternotomy with extrapericardial isolation), which also permits the comfort and surety of antegrade cerebral perfusion (if not involved in the dissected vessels). During distal circulatory arrest, antegrade cerebral perfusion at 10 mL/kg–1/min–1 is guaranteed through these lines to maintain a right radial artery pressure between 40 and 70 mmHg, monitored using near-infrared spectroscopy (NIRS). The presence of a significant reduction in regional cerebral oxygen saturation of one hemisphere indicates an insufficient perfusion, necessitating the addition of a direct antegrade perfusion of the left common carotid artery (8) (Figure 3).
To avoid bleeding following the direct cannulation of these vessels, we interpose an 8-10 mm Dacron graft prosthesis after partial clamping of the vessels. A Y-connector is inserted in the arterial line to allow distal antegrade systemic perfusion through the side branch of a multi-branched Dacron Graft prosthesis, to be started after the completion of the distal aortic anastomosis (9).
Spinal cord ischemia
A significant issue with FET is the occurrence of perioperative spinal cord ischemia, with an incidence of 1.3% to 25% (10,11) for both transient and permanent events. The handling and manipulation of the LSA is of vital importance. Antegrade perfusion with low-flow line of both subclavian arteries ensures an adequate spinal cord perfusion. However, as previously indicated, it is often very difficult to detach the subclavian artery from the aortic arch. Once extracorporeal circulation has been initiated, the origin of the subclavian artery is clamped and detached from the aortic arch. Subsequently, an 8-10 mm Dacron graft prosthesis is anastomosed in end-to-end fashion with 5-0 polypropylene. If the subclavian artery is particularly fragile, we advise the following: a Foley catheter 14-16 Fr (previously inserted through the Dacron graft prosthesis) is inserted in the LSA and gently inflated. In this manner, the anastomosis between the graft and LSA can be easily performed while allowing selective antegrade LSA perfusion.
Prosthesis delivery
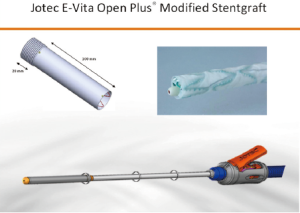
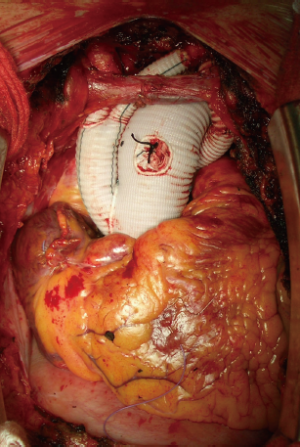
After aortic cross-clamping, cardioplegia is delivered and root or valve surgery with proximal anastomosis with a multi-branched graft prosthesis is performed. The subsequent delivery of the hybrid prosthesis in the arch and descending aorta is another important issue. The use of the Jotec E-vita Open Plus is now a frequent choice in many leading aortic centers. The hybrid stentgraft-vascular device is loaded in a delivery system with Squeeze-to-Release® mechanism for deployment. With a standard Seldinger technique, a guidewire is inserted, via transesophageal ultrasound, into the true aortic lumen for a greater stabilization of the device during the delivery (Figure 4). The original device provides a stent-graft portion length between 130 and 160 mm with a diameter of 24 to 40 mm and a length cuff of 70 mm. The coverage portion of the descending aorta down to vertebral level Th 8-9 has been suggested to correlate with a high incidence of spinal cord injuries. To reduce the frequency of this insidious complication, the device was further modified with a short distal stentgraft (100 mm) and a short proximal cuff (20 mm). Moreover, other new features were introduced: a small tip size for faster extraction, an external positioner aiding for precise realize and a short delivery system. In this way, markedly better handling with a more feasible, safer and faster delivery of the stentgraft in the downstream aorta has been obtained (Figure 5).
Visceral perfusion
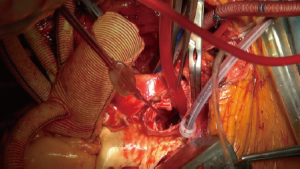
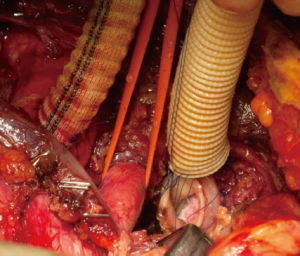
In October 2012, we introduced a new concept of distal aortic perfusion in order to significantly reduce the visceral ischemic time. After Jotec E-vita Open Plus delivery, a Pruitt Catheter is inserted into the stent-graft portion, with its terminal balloon filled with water to obtain an effective grip in the stent-graft. The Pruitt Catheter is then connected with a low-flow perfusion line to obtain a perfusion 700-1,000 mL/kg–1/min–1 of the distal thoracoabdominal aorta (Figure 6). Applying this technique, with associated systemic cooling to 26 °C and perfusion of both subclavian arteries, we have obtained a significant reduction of distal ischemic injury. Once the E-vita Open Plus Jotec has been implanted, the distal aortic neck is prepared using the Dacron cuff to strengthen the native aortic wall (Figure 7). The distal anastomosis between the new distal aortic neck and the multi-branched Dacron graft prosthesis is then performed with 3/0 polypropylene. We prefer a rerouting of epiaortic vessels, as in a beating heart approach, to reduce the time of selective cerebral perfusion and to ensure a more anatomic direction of the new epiaortic vessels, with an easier access to the bleeding points (Figure 8).
In conclusion, we have described some tips and tricks for the hybrid approach of TBAD with arch involvement, in which the management of cerebrospinal and visceral protection, aortic arch and distal aortic reconstruction with epiaortic vessels anastomosis are the most pertinent concerns. With the described approach, we demonstrate a simplified and thus reproducible process for an otherwise very time-consuming procedure.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Bünger CM, Kische S, Liebold A, et al. Hybrid aortic arch repair for complicated type B aortic dissection. J Vasc Surg 2013;58:1490-6. [PubMed]
- Geisbüsch P, Kotelis D, Müller-Eschner M, et al. Complications after aortic arch hybrid repair. J Vasc Surg 2011;53:935-41. [PubMed]
- Eggebrecht H, Thompson M, Rousseau H, et al. Retrograde ascending aortic dissection during or after thoracic aortic stent graft placement: insight from the European registry on endovascular aortic repair complications. Circulation 2009;120:S276-81. [PubMed]
- Cao P, De Rango P, Czerny M, et al. Systematic review of clinical outcomes in hybrid procedures for aortic arch dissections and other arch diseases. J Thorac Cardiovasc Surg 2012;144:1286-300, 1300.e1-2.
- Svensson LG, Kouchoukos NT, Miller DC, et al. Expert consensus document on the treatment of descending thoracic aortic disease using endovascular stent-grafts. Ann Thorac Surg 2008;85:S1-41. [PubMed]
- Jakob H, Tsagakis K, Pacini D, et al. The International E-vita Open Registry: data sets of 274 patients. J Cardiovasc Surg (Torino) 2011;52:717-23. [PubMed]
- Pacini D, Tsagakis K, Jakob H, et al. The frozen elephant trunk for the treatment of chronic dissection of the thoracic aorta: a multicenter experience. Ann Thorac Surg 2011;92:1663-70; discussion 1670.
- Urbanski PP, Lenos A, Kolowca M, et al. Near-infrared spectroscopy for neuromonitoring of unilateral cerebral perfusion. Eur J Cardiothorac Surg 2013;43:1140-4. [PubMed]
- Esposito G, Cappabianca G, Ciano M, et al. Mid-term results of the Lupiae technique in patients with De Bakey Type I acute aortic dissection. Eur J Cardiothorac Surg 2012;42:242-7; discussion 247-8. [PubMed]
- Misfeld M, Leontyev S, Borger MA, et al. What is the best strategy for brain protection in patients undergoing aortic arch surgery? A single center experience of 636 patients. Ann Thorac Surg 2012;93:1502-8. [PubMed]
- Etz CD, Luehr M, Kari FA, et al. Selective cerebral perfusion at 28 degrees C--is the spinal cord safe? Eur J Cardiothorac Surg 2009;36:946-55. [PubMed]