Acute type B aortic dissection: insights from the International Registry of Acute Aortic Dissection
Historical perspective
Aortic dissection is a catastrophic cardiovascular condition first described in detail by Frank Nicholls in his necropsy report of King George II (1). However, it was not until the mid-twentieth century that the first large case series was published (2) and the first surgical resection was performed (3). In 1965, DeBakey et al. identified clinically distinct variants of aortic dissection as type I and II originating in the ascending aorta and type III originating in the descending aorta (4), and that descending aortic dissection significantly differed from ascending aortic dissection with regards to presentation and outcomes. The Stanford classification was developed in the 1970s and it further highlighted the differing clinical practices in management of ascending vs. descending aortic dissection (5). However, data on the optimal diagnostic and treatment modalities for type III, also known as type B dissection, was slow to evolve throughout the latter half of the twentieth century even as newer diagnostic techniques and management strategies became commonplace.
Pathogenesis
The aorta, similar to other arteries, is composed of three layers from deep to superficial: intima, media, and adventitia. The intima, being in direct contact with blood, is a thin layer primarily composed of endothelial cells on a basement membrane. The media is the largest layer and is composed of muscle and connective tissue, while the adventitia is a thin layer of connective tissue (6). There are two primary hypotheses that have been proposed to explain acute aortic dissection. The first theory holds that an initial tear in the intima leads to blood from the aortic lumen surging into the media and separating the intima from the aorta and creating a true and false lumen. In contrast, the second theory holds that the vasa vasorum in the more outer portions of the media hemorrhage first, and then secondarily cause intimal rupture. In both theories, it is thought that the pressure of pulsatile blood flow extends the dissection, typically in an anterograde fashion (7). In 10-20% of acute aortic dissection cases, a variant such as an aortic intramural hematoma or penetrating atherosclerotic ulcer occurs (8).
International Registry of Acute Aortic Dissection
Aortic dissection remains a relatively rare but life-threatening disease in the current era, occurring in 3.5 per 100,000 persons per year (9) with recent evidence suggesting an increasing incidence of up to 14 per 100,000 persons per year (10). Up to 30% of these cases are believed to be type B in nature (11). To further elucidate contemporary practice patterns and outcomes of aortic dissection, The International Registry of Acute Aortic Dissection (IRAD) was established in 1996 initially enrolling patients in 12 centers of excellence in six countries with further expansion to 30 centers in 11 countries (Figure 1), and since then has enrolled over 5,000 patients. Since the landmark initial publication in 2000, IRAD publications have steadily increased improving knowledge and understanding of aortic dissection. Data from IRAD have identified basic risk factors including: bicuspid aortic valve, Marfan syndrome, male gender, age greater than 60, and hypertension (11).
With the large-scale IRAD study, comparisons regarding the demographics and clinical presentations of patients with type A versus type B disease also became possible. Patients with type B dissection are older, have higher rates of atherosclerosis, and are more likely to present with back pain rather than anterior chest pain (11). Importantly, half of type B dissections have an initial abnormal chest radiograph. Furthermore, the in-hospital mortality rate is 13% with the majority of deaths occurring within the first week of hospitalization. Independent predictors of death in type B dissection have been jointly termed the ‘deadly triad’: hypotension/shock, absence of chest/back pain on presentation, and branch vessel involvement (12). In recent years, analyses of IRAD data have gone beyond simply characterizing the patient with acute type B aortic dissection and have attempted to identify the means by which the outcome of such a patient could be improved. Thus, we present herein three areas in which IRAD data has recently advanced our understanding of acute type B aortic dissection.
Temporal classification
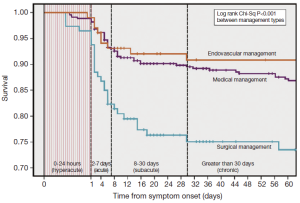
Classically, aortic dissection has been classified into two groups based upon on the time of symptom onset. Acute dissection has been diagnosed when clinical symptoms have lasted for 14 days or less, while greater than two weeks of symptom duration has been considered to be chronic. Although this distinction was made in an era prior to the advent of modern diagnostic and surgical and nonsurgical management strategies, this categorization has continued to guide contemporary care of aortic dissection. Recently, Booher et al. analyzed the IRAD database and developed Kaplan-Meier survival curves for type B aortic dissection identifying distinct inflection points and creating four distinct time periods: hyperacute (symptom onset to 24 hours), acute (2-7 days), subacute (8-30 days) and chronic (>30 days) (Figure 2). Cumulative survival continued to decline throughout all four of these temporal groups regardless of treatment modality: 94-99%, 82-93%, 77-92%, and 73-91% respectively (13). The finding that survival continues to decrease significantly, even up to 30 days post-symptom onset into what has been traditionally considered the chronic phase, is novel. While current guidelines for the management of patients with type B aortic disease utilize the traditional acute vs. chronic time periods with differing classification for various interventions in acute and chronic type B aortic dissection, it is expected that this new IRAD classification system will assist in improving ‘best practice’ for management (14).
Optimal treatment of aortic dissection in the 15-30 days from symptom onset time period remains uncertain. As such, the question remains of how to best treat patients presenting with subacute type B aortic dissection. The INSTEAD trial attempted to answer this question by randomizing patients with uncomplicated type B aortic dissection between 2 and 52 weeks from onset into either medical therapy or thoracic endovascular aortic repair (TEVAR) therapy. Though the study was initially found to be underpowered, 5-year all-cause mortality appeared favorable for TEVAR at 11.1% versus best medical therapy alone at 19.3% (P=0.13). Additionally, aorta-specific 5-year mortality was significantly lower in the TEVAR group at 6.9% versus 19.3% (P=0.04) (15). It appears that TEVAR, in addition to optimal medical therapy, is associated with delayed disease progression resulting in improved long-term survival. Akin et al. have suggested that instability in subacute or chronic type B dissection as evidenced by permanent thoracic pain, uncontrolled hypertension, or aortic diameter increasing beyond 55-60 mm (or at an annual rate >4 mm) would be appropriate indications for TEVAR intervention (16). However, in truth there remains paucity of data and our current views of best practices are likely transient. Future trials based upon the defined temporal subgroups are needed to further inform the optimal management and prognosis of acute vs. subacute vs. chronic type B aortic dissection.
Surveillance imaging for any type B aortic dissection is thought by many to provide benefit regardless of temporal or risk stratification or even treatment applied (17). In fact, the current 2010 American Heart Association (AHA)/American College Of Cardiology (ACC) guidelines for thoracic aortic disease call for CT or MR imaging of the thoracic aorta at discharge, 1 month, 3, 6, and 12 months postdissection with annual imaging thereafter, if stable (14). Unfortunately, contemporary epidemiological studies have shown that approximately 52% of patients are lost to follow-up by 28 months postdissection; such noncompliance with the guidelines is concerning as 38% of medically treated type B aortic dissection patients were found to have become complicated and required intervention during the follow-up period (18). Whether acute, subacute, or chronic, surveillance imaging should remain a cornerstone of optimal therapy.
Risk stratification
The complexities of management of type B acute dissection continue to remain a challenge. The classic understanding has been that type A and complicated type B acute aortic dissections require surgical resection while uncomplicated type B acute aortic dissection can be treated medically. More recently, emerging evidence from IRAD has begun to elucidate the risk stratification and management of such patients. While no uniform criteria exist to differentiate complicated versus uncomplicated type B acute dissection, a recent interdisciplinary consensus document has suggested the following definition of complicated type B acute aortic dissection: malperfusion indicated by impending organ failure, hypertension when associated with malperfusion or persisting with high levels despite full medical therapy, or increases in periaortic hematoma and hemorrhagic pleural effusion in two subsequent CT examinations suggestive of impending rupture (17). Per this definition, malperfusion is typically evidenced by clinical signs and/or laboratory markers. Typical clinical features may include: malperfusion of spinal arteries leading to paresis, and paraplegia or malperfusion of visceral arteries leading to abdominal pain. However, for those instances where malperfusion may remain too subtle to evoke clinical symptomology, laboratory markers provide a sensitive method of detection. Elevations in hepatic or pancreatic enzymes may similarly signify mesenteric or celiac malperfusion, while increased creatinine would signify renal malperfusion. Additionally, refractory pain may be a clinical symptom indicative of malperfusion as well. Indeed in IRAD, refractory pain has been noted to be a predictor of mortality increasing the risk of in-hospital mortality by over twenty-fold (35.6% vs. 1.5%, P=0.0003; OR 3.31, P=0.04) (19) (Figure 3).
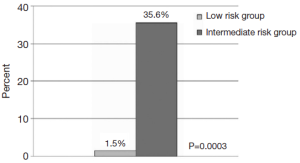
Literature from IRAD has shown that approximately 25% of patients presenting with acute type B aortic dissection meet the above definition for complicated status (20). In 2006, IRAD data reported a 3-year mortality for acute type B dissection approached nearly 1 in 4. Most interestingly, it was seen that acute type A dissection patients typically have early rates of high mortality that plateaued post-discharge. In contrast, acute type B dissection patients continue to have high rates of cumulative death in fact exceeding that of acute type A patients at 3 years. Whilst medical management has demonstrated an in-hospital mortality rate less than 10% (12), post-discharge the aortic disease continues to evolve eventually resulting in complicated type B disease. Typical criteria for complicated disease such as in-hospital shock, renal failure, and pleural effusion were found to be by and far the greatest predictors of 3-year mortality (21). Similarly, Trimarchi et al. showed in 2010 using IRAD data that acute type B aortic dissection patients with refractory hypertension (requiring > or =3 different classes of antihypertensive therapy at maximal tolerated doses) had a greater than 20-fold increase in mortality with medical management (35.6% vs. 1.5%; P=0.0003) (19). Unfortunately, the alternative of open surgical treatment carries an in-hospital mortality rate in excess of 30% (20).
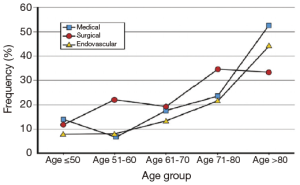
Similarly, complicated acute type B aortic dissection in the elderly shows even more striking mortality. Jonker et al. found that in IRAD the in-hospital mortality in such complicated patients greater than 70 years of age was found to be 30% vs. 10.1% for those younger than 70 treated with TEVAR, 34.2% vs. 17.2% for those treated surgically, and 32.2% vs. 14.2% for those treated with optimal medical management (Figure 4). Age greater than 70 was found to be an independent predictor for in-hospital mortality (OR 2.37, P=0.01) (22). Interestingly, although there was a significant decline in the rate of TEVAR or open surgical intervention in the elderly, there also was a non-significant trend towards decreased mortality in the elderly treated with TEVAR vs. open surgery or medical management. No doubt, complicated acute type B dissection carries excess mortality risk in all patients, though even more so in the elderly.
Thoracic endovascular aortic repair
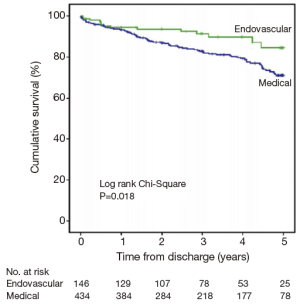
Based upon these observations, there has been a call for improved strategies for the acute management and surveillance of type B aortic dissection. Recently, Fattori et al. evaluated 5-year survival in type B aortic dissection patients enrolled in IRAD, receiving either only medical or TEVAR therapy (23). They found that approximately 1 in 4 patients with type B disease received TEVAR therapy. Moreover, those patients that received TEVAR therapy were far more likely to present with complicated disease (62% vs. 37%; P<0.001). Nonetheless, in-hospital mortality and one-year mortality was statistically similar between the two patient groups. Five-year mortality was found to be significantly lower in the group treated with TEVAR compared to optimal medical management alone (16% vs. 29%; P=0.018) (Figure 5). This benefit was seen despite the initially higher risk profile of the TEVAR group due to the complicated nature of their dissection. Thus, IRAD data suggests that a better 5-year outcome is seen in patients with type B complicated aortic dissection that are treated with TEVAR rather than medical management alone. Similarly, the INSTEAD-XL trial showed a 5-year all-cause mortality benefit with TEVAR compared to optimal medical management alone, driven primarily by aorta-related mortality (15). It appears that TEVAR therapy can modify the natural history of aortic disease without carrying an unacceptably higher procedure-related mortality risk in complicated type B dissection. The impact of TEVAR on uncomplicated type B dissection remains to be investigated. Although multiple, large randomized clinical trials are still lacking, IRAD data suggests that TEVAR therapy may be a promising therapy for appropriately selected patients. Future trials such as the Acute Dissection: stent graft or best medical therapy (ADSORB) trial are eagerly anticipated (24). Nonetheless, current standards-of-care remain: (I) medical therapy is the treatment of choice for uncomplicated acute type B aortic dissection; (II) TEVAR should be considered in complicated acute type B aortic dissection over open surgery (17).
Conclusions
While Frank Nicholls’ post-mortem description of King George II’s aortic dissection was written over two-and-half centuries ago, it was not until over a hundred years later that effective interventions were utilized. In contrast, the past two decades have brought along remarkable advancements in the understanding of acute aortic dissection that is beginning to transform management. Much of that progress has been and continues to be heralded by the IRAD group. Though many improvements have been made, the overall doctoring of acute aortic dissection remains suboptimal, and it is with great humility and optimism that we see a hopeful future.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Nicholls F. Observations concerning the Body of His Late Majesty, October 26, 1760, by Frank Nicholls, M. D. F. R. S. Physician to His Late Majesty. Philosophical Transactions 1761;52:265-75.
- Hirst AE Jr, Johns VJ Jr, Kime SW Jr. Dissecting aneurysm of the aorta: a review of 505 cases. Medicine (Baltimore) 1958;37:217-79. [PubMed]
- De Bakey ME, Cooley DA, Creech O Jr. Surgical considerations of dissecting aneurysm of the aorta. Ann Surg 1955;142:586-610; discussion, 611-2. [PubMed]
- Debakey ME, Henly WS, Cooley DA, et al. Surgical management of dissecting aneurysms of the aorta. J Thorac Cardiovasc Surg 1965;49:130-49. [PubMed]
- Daily PO, Trueblood HW, Stinson EB, et al. Management of acute aortic dissections. Ann Thorac Surg 1970;10:237-47. [PubMed]
- Kumar V, Abbas AK, Fausto N, et al. Robbins and Cotran pathologic basis of disease. 7th ed. Philadelphia: Elsevier Saunders, 2005:1525.
- Bonow RO, Mann DL, Zipes DP, et al. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine, Single Volume. 9th Edition. Amsterdam: Elsevier Health Sciences, 2011:2048.
- Tolenaar JL, Harris KM, Upchurch GR Jr, et al. The differences and similarities between intramural hematoma of the descending aorta and acute type B dissection. J Vasc Surg 2013;58:1498-504. [PubMed]
- Clouse WD, Hallett JW Jr, Schaff HV, et al. Acute aortic dissection: population-based incidence compared with degenerative aortic aneurysm rupture. Mayo Clin Proc 2004;79:176-80. [PubMed]
- Olsson C, Thelin S, Ståhle E, et al. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation 2006;114:2611-8. [PubMed]
- Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA 2000;283:897-903. [PubMed]
- Suzuki T, Mehta RH, Ince H, et al. Clinical profiles and outcomes of acute type B aortic dissection in the current era: lessons from the International Registry of Aortic Dissection (IRAD). Circulation 2003;108 Suppl 1:II312-7. [PubMed]
- Booher AM, Isselbacher EM, Nienaber CA, et al. The IRAD classification system for characterizing survival after aortic dissection. Am J Med 2013;126:730.e19-24.
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: executive summary. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Catheter Cardiovasc Interv 2010;76:E43-86. [PubMed]
- Nienaber CA, Kische S, Rousseau H, et al. Endovascular repair of type B aortic dissection: long-term results of the randomized investigation of stent grafts in aortic dissection trial. Circ Cardiovasc Interv 2013;6:407-16. [PubMed]
- Akin I, Kische S, Ince H, et al. Indication, timing and results of endovascular treatment of type B dissection. Eur J Vasc Endovasc Surg 2009;37:289-96. [PubMed]
- Fattori R, Cao P, De Rango P, et al. Interdisciplinary expert consensus document on management of type B aortic dissection. J Am Coll Cardiol 2013;61:1661-78. [PubMed]
- Kret MR, Azarbal AF, Mitchell EL, et al. Compliance with long-term surveillance recommendations following endovascular aneurysm repair or type B aortic dissection. J Vasc Surg 2013;58:25-31. [PubMed]
- Trimarchi S, Eagle KA, Nienaber CA, et al. Importance of refractory pain and hypertension in acute type B aortic dissection: insights from the International Registry of Acute Aortic Dissection (IRAD). Circulation 2010;122:1283-9. [PubMed]
- Fattori R, Tsai TT, Myrmel T, et al. Complicated acute type B dissection: is surgery still the best option?: a report from the International Registry of Acute Aortic Dissection. JACC Cardiovasc Interv 2008;1:395-402. [PubMed]
- Tsai TT, Fattori R, Trimarchi S, et al. Long-term survival in patients presenting with type B acute aortic dissection: insights from the International Registry of Acute Aortic Dissection. Circulation 2006;114:2226-31. [PubMed]
- Jonker FH, Trimarchi S, Muhs BE, et al. The role of age in complicated acute type B aortic dissection. Ann Thorac Surg 2013;96:2129-34. [PubMed]
- Fattori R, Montgomery D, Lovato L, et al. Survival after endovascular therapy in patients with type B aortic dissection: a report from the International Registry of Acute Aortic Dissection (IRAD). JACC Cardiovasc Interv 2013;6:876-82. [PubMed]
- Brunkwall J, Lammer J, Verhoeven E, et al. ADSORB: a study on the efficacy of endovascular grafting in uncomplicated acute dissection of the descending aorta. Eur J Vasc Endovasc Surg 2012;44:31-6. [PubMed]