Open surgical repair for chronic type B aortic dissection: a systematic review
Background
The optimal management of chronic type B aortic dissections (CBAD) remains controversial. Since the first widely published reports of endovascular stent-grating for descending aortic aneurysms in 1994 by Dake and colleagues (1), and its subsequent use in descending aortic dissections in 1999 (2,3), thoracic endovascular aortic repair (TEVAR) has supplanted open surgical repair (OSR) as the preferred treatment option for type B dissections. While there exists clear indications exist for TEVAR in acute complicated type B dissections, uncertainty persists regarding the superiority of TEVAR over OSR for CBAD (4-6).
The success of TEVAR is dependent on the thromboexclusion of the false lumen, as persistent false lumen perfusion can result in aortic expansions of up to 4 mm/year (7). In the chronic phase, there appears to be less consistent TEVAR-led aortic remodeling than in acute dissections, with total false lumen thrombosis ranging between 38-93%, and 15-17% of patients experiencing an increase in false lumen size in at least one location (8-11). While the short-term procedural benefits of an endovascular approach are undeniable compared to open surgery, its long-term advantage for CBAD is unclear. Indeed, a recent expert consensus on treatment of chronic dissections with endovascular stent-grafts concluded that TEVAR does not reduce aortic ruptures or increase life expectancy (4). OSR, on the other hand, eliminates the risk of aneurysm-related death in the treated segment. While historical surgical series have demonstrated high mortality rates (12-17), contemporary series, utilizing modern surgical techniques, report a positive trend towards more favorable patient outcomes.
The contemporary results of open surgery for type B dissections are critical to understanding the role of TEVAR in descending aortic pathologies, particularly given the unabated enthusiasm for endovascular interventions. The aim of the present study was to assess and summarize the outcomes of open surgical repair for chronic type B aortic dissections, with particular focus on contemporary data in the current endovascular era.
Methods
Literature search
Electronic searches were performed using Ovid Medline, Embase, Cochrane Central Register of Controlled Trials (CCTR), Cochrane Database of Systematic Reviews (CDSR), ACP Journal Club, and Database of Abstracts of Review of Effectiveness (DARE) from their date of inception to March 2014. To achieve the maximum sensitivity of the search strategy and identify all studies, we combined the terms: “aorta” and “dissection” and “chronic” as either key words or MeSH terms. The reference lists of all retrieved articles, as well as review articles, were reviewed for further identification of potentially relevant studies. All identified articles were systematically assessed using the inclusion and exclusion criteria.
Eligibility criteria
Eligible studies for the present systematic review included those in which patient cohorts underwent open surgery for chronic dissections of the descending aorta (Stanford type B or DeBakey type III dissection). Cases that involved the ascending aorta or aortic arch (type A dissections) or only the abdominal aorta were excluded. Studies were included regardless of the extent of the descending dissection. Chronicity was defined as greater than 14 days following symptomatic presentation or documentation of intimal entry tear.
Studies that did not include the predetermined primary endpoint of 30-day or in-hospital mortality were excluded. When institutions published duplicate studies with accumulating numbers of patients or increased lengths of follow-up, only the most complete reports were included for quantitative assessment at each time interval. Reports that presented primary endpoint data on 10 or more patients were included. All publications were limited to those involving human subjects and in the English language. Abstracts, case reports, conference presentations, editorials, and expert opinions were excluded. Review articles were omitted because of potential publication bias and duplication of results.
Data extraction and critical appraisal
All data were extracted independently from article texts, tables and figures by two investigators (R.P.D.S. and T.W.). Data was subsequently reviewed and tabulated by another investigator (D.H.T.). Discrepancies between the two reviewers were resolved by discussion and consensus with the third investigator (D.H.T.). Methodological quality was assessed using a 18-item validated quality appraisal checklist specifically developed for the evaluation of case series (18). Various elements of the study, including study objective, population, interventions, outcome measures, statistical analysis, results, conclusions, and competing interests were assessed. The final results were reviewed by the senior investigator (T.D.Y.).
Statistical analysis
Standard descriptive statistics were used to summarize demographic and baseline data of eligible patients. Data were presented as raw numbers with percentage or mean ± standard deviation as appropriate unless otherwise indicated. Pooled averages were estimated using the random-effects model proposed by DerSimonian and Laird (19). Pooled values were calculated for outcomes that were reported in at least 50% of studies and for at least 50% of total patients. Studies were further categorized into ‘pre-endovascular’ (historic series) and ‘endovascular’ era (contemporary series) based on whether more than half of its study period was after 1999, when the first series of stent-grafting for descending aortic dissections were widely reported (2,3).
Individual patient survival data was reconstructed using an iterative algorithm that was applied to solve the Kaplan-Meier equations originally used to produce the published graphs. This algorithm, as provided by Guyot and colleagues, uses digitalized Kaplan-Meier curve data to find numerical solutions to the inverted Kaplan-Meier equations (20). This algorithm assumes constant censoring and was calculated in R software (v.3.1.0). The reconstructed patient survival data were then aggregated to form combined survival curves.
Evidence of publication bias was sought using the methods of Egger et al. (21) and Begg et al. (22). If studies appear to be missing in areas of low statistical significance, then it is possible that the asymmetry is due to publication bias. If studies appear to be missing in areas of high statistical significance, then publication bias is a less likely cause of funnel asymmetry. Intercept significance was determined by the t-test suggested by Egger et al. All statistical analyses were conducted with Comprehensive Meta-analysis v2.2 (Biostat Inc, Englewood, NJ, USA). P values <0.05 were considered statistically significant.
Results
Quantity and quality of evidence
A total of 1,574 unique records were identified through electronic searches of the seven databases (Figure S1). After excluding records based on abstract, 225 full-text articles were assessed according to the inclusion and exclusion criteria. Nineteen relevant studies were included in the present review (2,14-17,23-36).
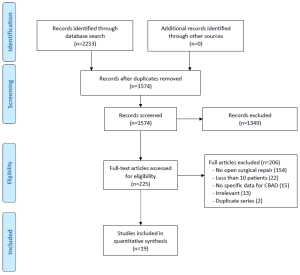
All of the included studies were retrospective observational series (Level 4 evidence) (Table 1). Only six series had greater than 50 patients (range, 79-169); the median size of studies was 35. Of the 19 studies, nine were categorized as being in the ‘endovascular era’ (contemporary series) (2,28,30-36), with only five studies that included patient cohorts solely after 1999. The landmark study by Nienaber et al. was included in the contemporary cohort as its patients were matched controls with the one of the first stent-grafting series at the same institution (2). One study published in 2008, which included a study period of 1983-2002, was excluded from the ‘endovascular era’ category as its follow-up data suggested the majority of cases were performed prior to 1999 (29).
Full table
Chronic dissection was explicitly defined as ≥2 weeks after symptomatic presentation in most studies, except one, which defined it as 3 weeks (15). Chronicity was not defined in 10 studies (2,24,26,27,29-32,34,36). All but two studies used the Stanford classification for aortic dissections (14,24,27).
The quality of the studies ranged from low to moderately high. Common limitations included recruitment from single-centers, failure to acknowledge competing interests, and lack of reporting on proportion lost to follow-up or length of follow-up.
Demographic and operative techniques
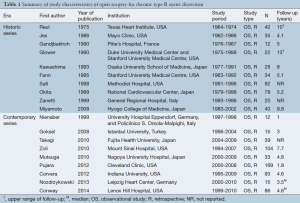
The demographic details are summarized in Table 2. In 19 studies, 970 patients underwent open surgery for CBAD. Overall, 76.5% of patients were male, with a weighted mean age of 57.9 years. Eighty-six percent of patients were hypertensive (2,17,23,25,30,31,33-36), 0-24% of patients had a history of stroke, while 0-33% of patients had pulmonary disease and 11.0% had renal dysfunction (2,16,17,23,28,30,31,33-36). Marfan syndrome was present in 12.0% of patients (2,17,23,26,28,30-33,35,36).
Full table
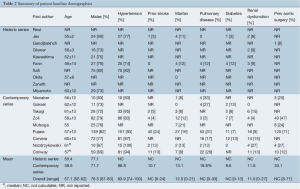
Surgical indication for the majority of cases included aneurysms ≥50 mm (27,34), >55 mm (30), >60 mm (26), unspecified growth rate or dilation limit (14,15,23,28,31,35), malperfusion (15,28,31,34,35), presence of symptoms (14,23,27,28,31,34,35), or rupture (26,31,35) (Table 3). Nine studies did not specify indications for surgery (2,16,17,24,25,29,32,33,36). In series from institutions that also reportedly performed TEVAR, indications for open repair included extensive involvement of the aorta (33,35) or connective tissue disease (35). Another study indicated that TEVAR was only a ‘complication-specific’ indication (28). The remaining six studies from the endovascular era did not provide any evidence that they also perform endovascular repairs (29-32,34,36), while another did not state indication for OSR (2).
Full table
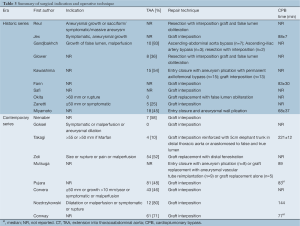
Thoracoabdominal involvement was identified in 48.5% of patients in contemporary studies, and 39.1% in historical series (overall, 45.8%). The majority of repairs involved graft replacement of the diseased aorta, while some only closed the entry tear and plicated the aneurysm. A wide range of spinal cord protection strategies were used, including CSF drainage, selective intercostal reimplantation and hypothermic circulatory arrest.
Mortality and morbidity
Early mortality at 30 days for the entire cohort was 11.1% (range, 0-33%) (Table 4). Stroke occurred in 5.6% of patients (range, 0-13%), while 4.9% of patients experienced spinal cord ischemia (range, 0-13%). Postoperative renal dysfunction affected 11.9% of patients (range, 0-33%). Subsequent re-exploration for bleeding was required for 9.9% of patients (range, 0-33%).
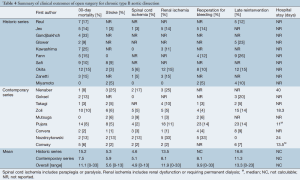
Full table
In contemporary studies, mortality was 7.5% compared to 15.2% for series in the pre-endovascular era. While the incidence of strokes and spinal cord ischemia was slightly higher for contemporary (stroke, 5.9% vs. 5.3%; SCI, 5.1% vs. 4.6%), these studies had better renal outcomes (8.1% vs. 13.5%).
Medium-term outcomes
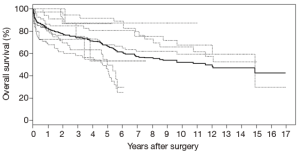
Individual patient survival data of 458 patients was reconstructed from the Kaplan-Meier survival curves of 7 studies (26,28-31,33,35). Analysis of this data using Kaplan-Meier methods demonstrate 1-, 2-, 3-, 5-, and 10-year survival of 82.1%, 77.1%, 74.1%, 66.3%, 50.8%, respectively (Figure 1). Late absolute reintervention rate was 13.3% for the entire cohort, but 11.3% for the studies in the endovascular era.
Publication bias
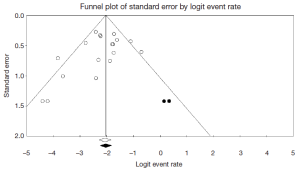
Begg’s rank correlation method (P=0.239) and Egger’s weighted (P=0.151) regression method were performed to assess publication bias in the literature. Although both tests suggest publication bias was not an influencing factor when mortality was selected as an outcome measure for all 19 included studies, visual inspection of the contour-enhanced funnel plot suggests small study effect exists (Figure S2). Using the imputed Trim and Fill method, the point estimate for mortality increased slightly from 11.1% to 11.7%.
Discussion
As management of acute aortic dissections improved over the years, there has been an increase in the number of patients with chronic dissections requiring treatment of late complications. Historically, complicated CBAD cases typically necessitated OSR, but since 1999, this has been preferentially supplanted by endovascular approaches. However, despite the lower operative morbidity and mortality of TEVAR, mid-term outcomes are less encouraging, with considerable rates of procedural failure due to endoleaks, persisting false lumen perfusion with aneurysmal dilatation, and need for reintervention (37-39). As such, recent debate has arisen over the current preference of endovascular approaches for the management of CBAD (4).
It is reasonable to expect the short-term procedural outcomes of a minimally invasive endovascular approach to be superior to that of open surgery. In the present series, OSR for CBAD resulted in an average 30-day mortality rate of 11.1% for the entire cohort and 7.5% for patients treated in the contemporary series. The present review also demonstrated that open surgery in the endovascular era carries considerable risks of postoperative strokes (5.9%), spinal cord ischemia (5.1%) and renal ischemia (8.1%). These poor outcomes may be partially attributable to patient selection and the extent of open surgery—48.5% of patients had sufficiently extensive disease to warrant thoracoabdominal repair, with some centers selectively reserving OSR for patients with extensive disease (33).
A key complication of open repair is the risk of spinal cord injuries. While the use of modern perfusion strategies, CSF drainage, active cooling, limitation of spinal cord collateral steal, maintenance of left subclavian artery patency, and close hemodynamic monitoring have improved outcomes, the risk of paraplegia is still not negligible (40). In the present review, 5.1% of patients in contemporary studies experienced some form of spinal cord injury. The identified studies used a variety of spinal cord protection techniques, which demonstrated limited understanding of optimal approach to minimize spinal cord injury.
The recent partiality for endovascular therapies has supplanted OSR as the preferred surgical approach for management of CBADs. As expected, the minimally invasive approach has reported favorable short-term outcomes, with 30-day mortality averaging between 0.8-3.2% in several systematic reviews that examined TEVAR use in chronic type B dissections (37,38,41). However, it must be noted that TEVAR is still a relatively new technique and, in the hands of inexperienced operators (≤20 patients experience), it can still result in mortality rates of up to 8.5% (37). Freedom from reintervention in TEVAR-treated CBAD patients has also been noted to be poor, ranging from 55-80% at 3 years (9,42-44), with reintervention rate of 15.9% (range, 0-60) (38). In a study using Medicare data in the United States of over 15,000 patients undergoing open or endovascular repair for descending thoracic aortic aneurysm, the authors concluded that despite short-term benefits, TEVAR was associated with poorer outcomes in the long-term compared to open surgery, a finding further confirmed with risk-adjusted and propensity-matched cohorts (45). Bearing in mind the limitations of analyzing Medicare data and the different patient sample compared to the present study, these findings nevertheless highlight the uncertainty associated with long-term outcomes of TEVAR treatments. Further investigation, with longer duration of follow-up with imaging, is required to provide greater understanding on this controversial matter.
The rapid advances in the management of chronic type B dissections reflect a growing necessity to conduct further research into this field. The role for OSR for chronic type B dissections needs to be clearly examined, as does patient selection criteria. Optimal timing between symptom onset and intervention must be further investigated, particularly as aortic remodeling is more likely to occur early in the dissection process (46,47). The role of stent-grafting of the descending aorta during open surgery for type A dissections also needs to be determined (48). Cost analysis and quality of life assessment, factors not examined in any of the included studies in this review, must also be better understood. With the formal approval of endoprostheses for use in type B dissections last year by the Food and Drugs Administration in the United States, there will undoubtedly be an upsurge in the use of TEVAR, thus greatly driving the impetus for further research in this field. For patients, the trade-off between the long-term complications of TEVAR, including the risk of endoleaks, further aneurysmal dilation, and the need for ongoing follow-up, must also be carefully considered on a case-by-case basis against the risks of open surgery.
The results of the present review were limited by the heterogeneous nature of the patient cohorts, particularly with respect to indication for surgery and the extent of repair. Direct comparisons to TEVAR should also be made with caution, as TEVAR was typically preferred for those with limited disease and with suitable proximal and distal landing zones, and those considered high risk for open surgery. The lack of higher-level evidence, compounded by heterogenous definitions, surgical techniques, and follow-up protocols, also curtailed robust analysis of the data. Absence of raw patient data meant that long-term survival outcomes could only be estimated using statistical aggregation methods from published Kaplan-Meier graphs. Such techniques assume constant censoring, and also combine what can be a rather heterogenous patient cohort. Finally, while the majority of studies in the present review were performed at centers where there was no endovascular alternative, the preference of TEVAR as the first-line surgical treatment in some centers has relegated open surgery for those with connective tissue or extensive disease, and those unsuitable for endovascular intervention (33,35,49).
In conclusion, OSR for chronic type B dissection remains an important approach for patients. The short-term outcomes of modern open surgery is acceptable, although they appear poorer compared to TEVAR. More research is required to determine long-term benefits of open surgery and TEVAR, as well as the appropriate indications for either approach.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Dake MD, Miller DC, Semba CP, et al. Transluminal placement of endovascular stent-grafts for the treatment of descending thoracic aortic aneurysms. N Engl J Med 1994;331:1729-34. [PubMed]
- Nienaber CA, Fattori R, Lund G, et al. Nonsurgical reconstruction of thoracic aortic dissection by stent-graft placement. N Engl J Med 1999;340:1539-45. [PubMed]
- Dake MD, Kato N, Mitchell RS, et al. Endovascular stent-graft placement for the treatment of acute aortic dissection. N Engl J Med 1999;340:1546-52. [PubMed]
- Svensson LG, Kouchoukos NT, Miller DC, et al. Expert consensus document on the treatment of descending thoracic aortic disease using endovascular stent-grafts. Ann Thorac Surg 2008;85:S1-41. [PubMed]
- Fattori R, Cao P, De Rango P, et al. Interdisciplinary expert consensus document on management of type B aortic dissection. J Am Coll Cardiol 2013;61:1661-78. [PubMed]
- Moulakakis KG, Mylonas SN, Dalainas I, et al. Management of complicated and uncomplicated acute type B dissection. A systematic review and meta-analysis. Ann Cardiothorac Surg 2014;3:234-46. [PubMed]
- Sueyoshi E, Sakamoto I, Hayashi K, et al. Growth rate of aortic diameter in patients with type B aortic dissection during the chronic phase. Circulation 2004;110:II256-61. [PubMed]
- Patterson BO, Cobb RJ, Karthikesalingam A, et al. A systematic review of aortic remodeling after endovascular repair of type B aortic dissection: methods and outcomes. Ann Thorac Surg 2014;97:588-95. [PubMed]
- Sayer D, Bratby M, Brooks M, et al. Aortic morphology following endovascular repair of acute and chronic type B aortic dissection: implications for management. Eur J Vasc Endovasc Surg 2008;36:522-9. [PubMed]
- Tolenaar JL, Kern JA, Jonker FH, et al. Predictors of false lumen thrombosis in type B aortic dissection treated with TEVAR. Ann Cardiothorac Surg 2014;3:255-63. [PubMed]
- Kusagawa H, Shimono T, Ishida M, et al. Changes in false lumen after transluminal stent-graft placement in aortic dissections: six years’ experience. Circulation 2005;111:2951-7. [PubMed]
- Miller DC, Mitchell RS, Oyer PE, et al. Independent determinants of operative mortality for patients with aortic dissections. Circulation 1984;70:I153-64. [PubMed]
- DeBakey ME, McCollum CH, Crawford ES, et al. Dissection and dissecting aneurysms of the aorta: twenty-year follow-up of five hundred twenty-seven patients treated surgically. Surgery 1982;92:1118-34. [PubMed]
- Reul GJ, Cooley DA, Hallman GL, et al. Dissecting aneurysm of the descending aorta. Improved surgical results in 91 patients. Arch Surg 1975;110:632-40. [PubMed]
- Gandjbakhch I, Jault F, Vaissier E, et al. Surgical treatment of chronic aortic dissections. Eur J Cardiothorac Surg 1990;4:466-71. [PubMed]
- Glower DD, Fann JI, Speier RH, et al. Comparison of medical and surgical therapy for uncomplicated descending aortic dissection. Circulation 1990;82:IV39-46. [PubMed]
- Fann JI, Smith JA, Miller DC, et al. Surgical management of aortic dissection during a 30-year period. Circulation 1995;92:II113-21. [PubMed]
- Moga C, Guo B, Schopflocher D, et al. Development of a quality appraisal tool for case series studies using a modified Delphi technique. Available online: http://www.ihe.ca/documents/Case%20series%20studies%20using%20a%20modified%20Delphi%20technique.pdf
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. [PubMed]
- Guyot P, Ades AE, Ouwens MJ, et al. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol 2012;12:9. [PubMed]
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [PubMed]
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088-101. [PubMed]
- Jex RK, Schaff HV, Piehler JM, et al. Early and late results following repair of dissections of the descending thoracic aorta. J Vasc Surg 1986;3:226-37. [PubMed]
- Kawashima Y, Shirakura R, Nakano S, et al. Long-term results of entry closure and aneurysmal wall plication with axillofemoral bypass: a new procedure for repair of DeBakey type 3 dissecting aneurysm. Surgery 1993;113:59-64. [PubMed]
- Safi HJ, Miller CC 3rd, Reardon MJ, et al. Operation for acute and chronic aortic dissection: recent outcome with regard to neurologic deficit and early death. Ann Thorac Surg 1998;66:402-11. [PubMed]
- Okita Y, Tagusari O, Minatoya K, et al. Is distal anastomosis only to the true channel in chronic type B aortic dissection justified? Ann Thorac Surg 1999;68:1586-91. [PubMed]
- Zanetti PP, Sorisio V, Rosa G, et al. Type III dissection according to DeBakey. Comments on 45 cases treated. J Cardiovasc Surg (Torino) 1999;40:111-6. [PubMed]
- Goksel OS, Tireli E, Kalko Y, et al. Mid-term outcome with surgery for type B aortic dissections: a single center experience. J Card Surg 2008;23:27-30. [PubMed]
- Miyamoto Y, Ohata T, Mitsuno M, et al. Long-term outcomes after entry closure and aneurysmal wall plication for type B aortic dissection. Eur J Cardiothorac Surg 2008;33:152-6. [PubMed]
- Takagi Y, Ando M, Higuchi Y, et al. Recent outcomes of surgery for chronic type B aortic dissection. Ann Vasc Dis 2010;3:215-21. [PubMed]
- Zoli S, Etz CD, Roder F, et al. Long-term survival after open repair of chronic distal aortic dissection. Ann Thorac Surg 2010;89:1458-66. [PubMed]
- Mutsuga M, Narita Y, Araki Y, et al. Spinal cord protection during a thoracoabdominal aortic repair for a chronic type B aortic dissection using the aortic tailoring strategy. Interact Cardiovasc Thorac Surg 2010;11:15-9. [PubMed]
- Pujara AC, Roselli EE, Hernandez AV, et al. Open repair of chronic distal aortic dissection in the endovascular era: Implications for disease management. J Thorac Cardiovasc Surg 2012;144:866-73. [PubMed]
- Corvera JS, Fehrenbacher JW. Open repair of chronic aortic dissections using deep hypothermia and circulatory arrest. Ann Thorac Surg 2012;94:78-81; discussion 82-3. [PubMed]
- Nozdrzykowski M, Etz CD, Luehr M, et al. Optimal treatment for patients with chronic Stanford type B aortic dissection: endovascularly, surgically or both? Eur J Cardiothorac Surg 2013;44:e165-74; discussion e174.
- Conway AM, Sadek M, Lugo J, et al. Outcomes of open surgical repair for chronic type B aortic dissections. J Vasc Surg 2014;59:1217-23. [PubMed]
- Eggebrecht H, Nienaber CA, Neuhäuser M, et al. Endovascular stent-graft placement in aortic dissection: a meta-analysis. Eur Heart J 2006;27:489-98. [PubMed]
- Thrumurthy SG, Karthikesalingam A, Patterson BO, et al. A systematic review of mid-term outcomes of thoracic endovascular repair (TEVAR) of chronic type B aortic dissection. Eur J Vasc Endovasc Surg 2011;42:632-47. [PubMed]
- Andersen ND, Keenan JE, Ganapathi AM, et al. Current management and outcome of chronic type B aortic dissection: results with open and endovascular repair since the advent of thoracic endografting. Ann Cardiothorac Surg 2014;3:264-74. [PubMed]
- Wan IY, Angelini GD, Bryan AJ, et al. Prevention of spinal cord ischaemia during descending thoracic and thoracoabdominal aortic surgery. Eur J Cardiothorac Surg 2001;19:203-13. [PubMed]
- Amano J, Kuwano H, Yokomise H. Thoracic and cardiovascular surgery in Japan during 2011: Annual report by The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2013;61:578-607. [PubMed]
- Böckler D, Schumacher H, Ganten M, et al. Complications after endovascular repair of acute symptomatic and chronic expanding Stanford type B aortic dissections. J Thorac Cardiovasc Surg 2006;132:361-8. [PubMed]
- Nienaber CA, Kische S, Rousseau H, et al. Endovascular repair of type B aortic dissection: long-term results of the randomized investigation of stent grafts in aortic dissection trial. Circ Cardiovasc Interv 2013;6:407-16. [PubMed]
- Kang WC, Greenberg RK, Mastracci TM, et al. Endovascular repair of complicated chronic distal aortic dissections: intermediate outcomes and complications. J Thorac Cardiovasc Surg 2011;142:1074-83. [PubMed]
- Goodney PP, Travis L, Lucas FL, et al. Survival after open versus endovascular thoracic aortic aneurysm repair in an observational study of the Medicare population. Circulation 2011;124:2661-9. [PubMed]
- Qing KX, Yiu WK, Cheng SW. A morphologic study of chronic type B aortic dissections and aneurysms after thoracic endovascular stent grafting. J Vasc Surg 2012;55:1268-75; discussion 1275-6. [PubMed]
- Stanley GA, Murphy EH, Knowles M, et al. Volumetric analysis of type B aortic dissections treated with thoracic endovascular aortic repair. J Vasc Surg 2011;54:985-92; discussion 992. [PubMed]
- Tian DH, Wan B, Di Eusanio M, et al. A systematic review and meta-analysis on the safety and efficacy of the frozen elephant trunk technique in aortic arch surgery. Ann Cardiothorac Surg 2013;2:581-91. [PubMed]
- Geisbüsch P, Kotelis D, von Tengg-Kobligk H, et al. Thoracic aortic endografting in patients with connective tissue diseases. J Endovasc Ther 2008;15:144-9. [PubMed]





