Video-assisted thoracoscopic pulmonary resections - The Melbourne experience
Background: Despite its privileged economic and educational place in the world, Melbourne was relatively slow to embrace video-assisted thoracic surgery (VATS) for lobectomy. The initial driver of this was Professor C Peter Clarke at the Austin Hospital at the beginning of the new millennium. His legacy was carried on by his apprentice, but at St Vincent’s Hospital. After a period of slow development, it became the procedure of choice from 2005, and began to filter sporadically to other hospitals from 2010.
Methods: This paper details the historical development, techniques and results of 343 VATS pulmonary resections (including lobectomies, sub-lobar anatomical resections, sleeve resections, bi-lobectomies and pneumonectomies).
Results: In-hospital and 30-day mortality was 2.0% and 5-year survival for all stages of NSCLC was 70%. Over 36% of patients were stage II-III using the new 7th revision TNM staging system. The conversion to thoracotomy rate was 4.7%. The estimated learning curve for this experience VATS lobectomy appears to be in the range of 15-20 cases. In this series, the same lymph node dissection or sampling was attempted and usually achieved as would have occurred at thoracotomy.
Conclusions: The results confirm the findings of other large case series that the benefits of a minimally invasive approach are achieved without compromising the long-term survival.
Key words: Video-assisted thoracic surgery; pulmonary resection; lung cancer; Melbourne experience
Introduction
Prologue - history of VATS lobectomy in melbourne: The austin years
Advanced thoracoscopic techniques were introduced to Melbourne, ostensibly by Professor C. Peter Clarke and his specialist Thoracic Surgical Unit at the Austin Hospital, in the early 1990s. Even at this time, there appeared to be no inclination, suggestion or prediction that a full lobectomy and node dissection by video-assisted thoracic surgery (VATS) would ever be considered. Although progressive in thoracoscopic techniques generally (e.g., oesophagectomy, sympathectomy, complex wedge resection), Melbourne was quite late in uptake of VATS Lobectomy by the standards of other centres around the world. At this time in the US, Kirby and colleagues in Cleveland, Dallas and Pittsburgh were working through that country’s initial experience (1). Simultaneously in Taiwan, Liu and his colleagues at Chang Gung Memorial Hospital began performing VATS Lobectomy without the benefit of vascular staplers (2). McKenna also began his now enormous experience around this time (3), as did Walker in Edinburgh (4).
After 1999, Clarke instructed his senior registrar to perform exploratory thoracoscopy on every case of peripheral NSCLC as he was searching for the perfect case for a “VATS lobectomy”. Eventually a 72 year-old female ex-smoker with a left lower lobe peripheral adenocarcinoma was identified with a wide-open complete fissure. After offering the advice that the registrar should make use of a “large-ish” incision from the outset (rather than extending a small incision at the end of the case to retrieve the specimen), he left the room to finish some dictating. By the time Clarke returned, the struggling registrar had dissected and tied the artery and divided the vein with an endoscopic stapler via a 5 cm anterior incision and two VATS ports. At this point, Clarke drily remarked that he had intended for the registrar to perform a normal thoracotomy incision, just without rib-spreading! Using the stapler normally used for bullectomy, the left lower lobe bronchus was divided after checking that the upper lobe still inflated. Retrieval of the lung was not too difficult being only a 2 cm tumour. The patient went home on day 5 without complication. On review two weeks later the patient was highly mobile, and grateful that she had been able to return to her ballroom dancing almost straight away. Despite this success, it was to be many years before VATS Lobectomy became a common operation at any hospital in Melbourne.
St Vincent’s Hospital - The learning curve
After completion of the fellowship at Austin Hospital, the author’s appointment at St Vincent’s Hospital commenced in 2001. This move coincided with the retirement of Professor Clarke, effectively ending the Austin Hospital VATS Lobectomy programme for the next 10 years. From 2001-2005, using the selection process inherited from Clarke, 13 VATS lobectomies were attempted at St Vincent’s Hospital. One had to be converted due to bleeding from the left superior segmental pulmonary artery branch of the left lower lobe. Another required an unexpected limited chest wall resection, but was still completed without rib-spreading. As a result of continuing Clarke’s selection policy all but one case was a lower lobectomy. The results were encouraging. The median post-operative stay was six days - one day less than our thoracotomies - with a single outlier of 43 days and no operative mortality. All, except perhaps one case (a carcinoid), were for malignant indications.
The single case of upper lobectomy was late in 2004. A young male patient required a metastasectomy for a small sarcoma metastasis deep in his right upper lobe. The plan had been to perform a hand-assisted thoracoscopic wedge resection (), but after palpation, it was a little too deep. He had an unusually well formed horizontal fissure at exploration; therefore an attempt was made at VATS right upper lobectomy. At this time the author had become acquainted with the techniques of Rice, D’Amico and McKenna through various conferences and publications. Dividing structures from anterior to posterior, this was successful. It was time to widen the indications for this surgery.
Thoracic surgery sub-specialization at St Vincent’s
Thoracic workload increased markedly at St Vincent’s Hospital from 2000-2005. Following the recruitment of a VATS lobectomy-trained thoracic surgeon from Memorial Hospital in New York in 2005, it was possible to expand the VATS lobectomy program exponentially at both the public and private campuses of St Vincent’s hospital. The technique became a very reproducible procedure, with only selected cases receiving thoracotomy, rather than the reverse. Being at a major referral centre for sarcoma and colorectal carcinoma, as well as having cross-appointment with Peter MacCallum Cancer Centre, there was a significant metastasectomy referral practice, which meant every type of sub-lobar anatomical resection had to be added to the VATS lobectomy repertoire. The rate of VATS lobectomy subsequently increased from three or four to an average of 50 per year, and an in-house wet lab course was designed for surgeons to transition from open to VATS lobectomy. The methods and results of over a decade of VATS lobectomy development in Melbourne are detailed in this paper.
Methods
Patient selection
All patients with a tumour of short axis diameter <5 cm were considered eligible for exploratory thoracoscopy and trial dissection, unless they had clear invasion of mediastinal structures or required sleeve resection. From 2008, sleeve resection of the bronchus was no longer considered a contra-indication in the absence of malignant fixed hilar nodes. Revision surgery, hilar node involvement and adhesions that could result in lengthy or bloody dissections were considered a high risk for conversion.
Surgical technique
Patient positioning: The patient is placed into the appropriate lateral position with the exception that both legs are bent at the knee and the hip pulled posteriorly and the shoulder pushed anteriorly. A table break at the waist is used to widen the intercostal spaces and flatten the hip out of the operative field. This manoeuvre is especially useful in females, as the hip can impede movement of the camera head on the telescope. Standard preparation and draping is then performed as for a thoracotomy.
Port placement: Usually the first port is placed in the 7th interspace, mid axillary line, for exploratory thoracoscopy. For the posterior port, the surface marking is the 8th interspace, just posterior to the posterior axillary line. However, the thoracoscopic view is generally used to determine a location vertically above the free edge of the collapsed lower lobe. These ports are moved posteriorly 1-2 cm for the left-sided approach due to the presence of the left ventricle.
The location of the utility incision should always be determined by thoracoscopy, unless adhesions preclude this manoeuvre. From the mid to anterior axilla region, a long needle is used to localize the interspace that allows a perpendicular drop directly to the superior pulmonary vein. This interspace is optimal for upper and middle lobectomies, and the interspace below is optimal for lower lobectomies. If the interspace does not quite line up with the vein, the more cephalad interspace is chosen. In practice, this is usually the 4th interspace and the incision extends 4-5 cm from mid to anterior axillary lines, between the free edges of latissmus dorsi and pectoralis major.
A standard 10-12 mm port is used for the thoracoscope. An XS or XXS Alexis retractor (Applied Medical) is used to protect the posterior 20-25 mm port. A small rigid Alexis retractor is used to retract and protect the utility incision in the axilla. The large chest wall muscles do not have to be divided extensively as the Alexis retractor stretches up the defect atraumatically and provides a good working incision. Internally, the intercostal muscles can be divided more widely with diathermy to allow passive rib spreading and facilitate specimen removal.
Order of dissection: For upper and middle lobes, the appropriate pulmonary vein tributary is dissected and divided initially with an endoscopic vascular stapler. The truncus artery is divided next for upper lobectomy, or the middle lobe artery for a middle lobectomy. Division of the bronchus or the remaining pulmonary artery branches is performed depending on their accessibility and the completeness of the fissure. The fissure is divided last with an endoscopic stapler to prevent air leak.
Throughout the procedure, hilar and mediastinal lymph nodes are dissected and removed to aid the skeletonization of the hilar structures. This is best done prior to division of the next structure. Any unsampled or undissected lymph nodes stations are cleared after removal of the lobe in an EndoCatch™ (Covidien®) specimen retrieval bag.
Larger tumours may necessitate extension of the utility incision, or at least further division internally of the intercostal muscles to allow greater rib space separation.
Closure: A 28 French intercostal catheter is placed through the thoracoscope port site and secured with heavy silk. A figure of eight absorbable suture is adequate for the deep tissues of the posterior port. The utility incision is closed in layers starting with serratus anterior then latissmus dorsi. No attempt is made to close the intercostal muscles or re-approximate the ribs. Skin is then re-apposed with an absorbable subcuticular suture.
Statistical analysis
A retrospective analysis of prospectively collected dataset was performed. Perioperative morbidity and mortality results were tabulated and overall survival was calculated using the Kaplan Meier method.
Results
Between 2001 and 2012, 343 major pulmonary resections were undertaken by VATS. This included 257 lobectomies, 63 segmentectomies or segment-sparing lobectomies, 13 pneumonectomies, 6 bilobectomies, 4 sleeve resections. Patient ages ranged from 15-91 years with a median age of 67 years. For NSCLC, the median age was 70. Of the malignant pathologies, 236 cases were for NSCLC, 63 for metastasectomy, 22 for carcinoid, five for SCLC, and one case was for lymphoma. Benign diagnoses were found in 16 and included mycobacterial abscess, sequestration, bronchiolitis obliterans with obstructive pneumonia, bronchiectasis, amyloid, aspergilloma, bullitis, hamartoma and sarcoid.
For NSCLC, tumours ranged from 4 mm to 10 cm in diameter (including lepidic component), with a median of 25 mm. Using the 7th revision of the TNM system, the stage spread showed more than half of patients as stage IA or IB (Table 1). The median post-operative length of stay was 6 days (range, 2-48 days). There were 16 conversions (4.7%) for extensive adhesions, failure to progress, severe intra-operative haemorrhage or need for complex resection or reconstruction of a mediastinal or hilar structure.
Complications requiring prolonged admission, re-admission, re-operation or admission to ICU are listed in Table 2. Those occurring at a rate of more than 1% were prolonged air leak (19, 5.5%), pneumonia (18, 5.2%), intra-operative bleed from pulmonary artery branch (7, 2.0%) and re-operation for haemorrhage (4, 1.2%). The 30-day mortality rate and in-hospital mortality rate were both 2.0%.
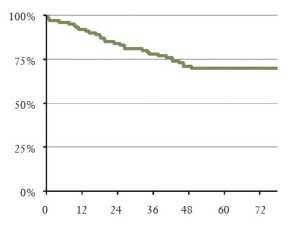
Overall survival for the whole cohort was 73% at 5 years, and for NSCLC, the overall survival was 70% (Figure 1).
| Table 1 Stage spread of VATS Lobectomy NSCLC cases using the 7th Revision of the TNM system | ||
| Stage | n | % |
| 1a | 83 | 35 |
| 1b | 67 | 29 |
| 2a | 20 | 8.5 |
| 2b | 24 | 10 |
| 3a | 33 | 14 |
| 3b/4 | 9 | 3.5 |
| TOTAL | 236 | 100 |
| Table 2 List of adverse events resulting in prolonged admission, re-admission, re-operation or admission to intensive care unit (Grade 3 Complications) | ||
| Adverse event* | n | % |
| Prolonged air leak | 19 | 5.5 |
| Pneumonia | 18 | 5.2 |
| Bleed from PA | 7 | 2.0 |
| Empyema | 5 | 1.5 |
| Re-op for bleeding | 4 | 1.2 |
| Sputum retention | 3 | 0.9 |
| SVT | 3 | 0.9 |
| NSTEMI | 3 | 0.9 |
| Bronchopleural fistula | 2 | 0.6 |
| Chyle leak | 2 | 0.6 |
| PA division | 2 | 0.6 |
| RML bronchus division | 2 | 0.6 |
| CVA | 2 | 0.6 |
| Acute tubular necrosis | 2 | 0.6 |
| Urinary retention | 1 | 0.3 |
| Bleed from left atrium | 1 | 0.3 |
| Bleed from Aortic branch | 1 | 0.3 |
| Urinary tract infection | 1 | 0.3 |
| Aspiration | 1 | 0.3 |
| Pulmonary hypertension | 1 | 0.3 |
| Delirium | 1 | 0.3 |
| ARDS | 1 | 0.3 |
| *Some patients had several complications so the number of adverse events exceeds the number of patients with adverse events. PA = pulmonary artery; SVT = supraventricular tachyarrhythmia; NSTEMI = non-ST elevation myocardial infarction; RML = right middle lobe; CVA = cerebrovascular accident; ARDS = adult respiratory distress syndrome | ||
Discussion
Despite being a relatively late adopter, by the standards of the major international VATS lobectomy centres, St Vincent’s Hospital in Melbourne rapidly transitioned to a VATS Lobectomy-predominant practice including complex sub-lobar and reconstructive techniques. This trend has now spread to other centres in Melbourne. This has happened despite the reticence of the majority of thoracic surgery centres in Australia (and around the World) to develop the technique. The Surveillance, Epidemiology, and End-Results Medicare Database showed that between 1995 and 2002, the rate of VATS Lobectomy only increased from 1% to 9% (6). From then to 2007, using the more specialty-specific Society of Thoracic Surgeons Database, the rate of VATS lobectomy was still only 20%. One possible cause for this low rate is the difficulty in introducing these techniques in busy open-heart surgery units. Thoracic surgery tends to be relegated to filling empty lists, and few surgeons have the time to conduct research or up-skill and retrain in new thoracic techniques. This will perhaps be solved by a new generation of surgeons that are used to laparoscopic and thoracoscopic techniques as routine procedures. In our experience of training future thoracic surgeons, the enhanced visualization at VATS lobectomy offers advantages in teaching the anatomy and dissection for both open and thoracoscopic approaches.
The learning curve at St Vincent’s Hospital lasted for about five years, partly due to a reluctance to break with the traditional approach to lobectomy, namely dissecting the artery from the fissure. In terms of case numbers, our learning curve was estimated to be between 15 and 20 cases. This is consistent with a recent robotic VATS lobectomy publication that determined that the learning curve amounted to 18 cases based on operative time and conversion rate (7). A previous publication estimated the learning curve for VATS lobectomy was 25 cases (8).
The St Vincent’s Hospital results have been continually self-audited and longer-term survival monitored to ensure that at least as good results are being achieved as with open surgery prior to 2005. This is a pre-requisite for introducing a new variation of a technique. Despite the oft-quoted potential bleeding difficulties, which we indeed encountered, we believe that our results now justify this significant change in our practice.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Kirby TJ, Rice TW. Thoracoscopic lobectomy. Ann Thorac Surg 1993;56:784-6.
- Liu HP, Chang CH, Lin PJ, et al. Thoracoscopic-assisted lobectomy. Preliminary experience and results. Chest 1995;107:853-5.
- McKenna RJ Jr. Lobectomy by video-assisted thoracic surgery with mediastinal node sampling for lung cancer. J Thorac Cardiovasc Surg 1994;107:879-81;discussion 881-2.
- Walker WS, Carnochan FM, Pugh GC. Thoracoscopic pulmonary lobectomy. Early operative experience and preliminary clinical results. J Thorac Cardiovasc Surg 1993;106:1111-7.
- Wright GM, Clarke CP, Paiva JM. Hand-assisted thoracoscopic surgery. Ann Thorac Surg 2003;75:1665-7.
- Farjah F, Wood DE, Mulligan MS, et al. Safety and efficacy of video-assisted versus conventional lung resection for lung cancer. J Thorac Cardiovasc Surg 2009;137:1415-21.
- Veronesi G, Agoglia BG, Melfi F, et al. Experience With Robotic Lobectomy for Lung Cancer. Innovations (Phila) 2011;6:355-360.
- Chin CS, Swanson SJ. Video-assisted thoracic surgery lobectomy: centers of excellence or excellence of centers? Thorac Surg Clin 2008;18:263-8.